Abstract
Abstract 2829
Peripheral T/NK cell lymphomas (PTCL) have a dismal prognosis with an overall 5yr survival < 30% and of 21% and 6% for intermediate-high and high IPI scores, respectively. The incorporation of new agents and transplant procedures in upfront strategies is a present challenge to investigators, due to the advanced age of the patient population and to the unsatisfactory results achieved with anthracyclines- based standard or intensified regimens. The nucleoside analog gemcitabine has shown high single agent activity in recurrent disease, but very little data are available on the upfront efficacy of gemcitabine-based combinations. We assessed activity, toxicity and stem cells (SCs) mobilizing capacity of a dose-dense combination of gemcitabine (G) with ifosfamide (Ifo) and oxaliplatin (Ox) (GIFOX regimen) for front-line therapy of PTCL other than anaplastic large cell subtypes and with 3–5 IPI scores.
Patients had to receive 6 GIFOX courses at biweekly intervals; SCs mobilization and consolidation of chemosensitive response with autologous transplantation (ASCT) were planned after the 3rd and the 6th course, respectively. GIFOX consisted of G 1200 mg/m2 D1, Ox 120 mg/m2 D2 and Ifo 5 g/m2 D2, as a 24h single infusion in pts aged ≤ 65 years, or fractionated over days 2, 3 and 4 in pts aged > 65 years, G-CSF 5 mcg/kg/d DD 7–11 (10 mcg/kg/d, 3rd course until SCs mobilization). No dose reductions were planned in case of inadequate bone marrow recovery, and treatment was delayed until the absolute neutrophil count was more than 1000/μL and the platelet count more than 50000/μL. For patients > 65 years the agents dosing was determined according to the nadir neutrophil or platelet counts with reduction to 75% of the planned dose for gemcitabine in case of CTCAE v.3 grade 3 toxicity, and for all the three drugs in case of grade 4 toxicity. A strict monitoring of Clcr was required and Ifo doses were reduced according to Kintzel (Cancer Treat Rev, 1995, 21(1):33-64).
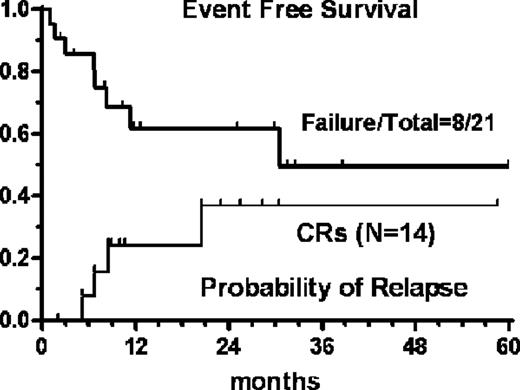
Twenty-one pts (M/F=15/6; median age 63 yrs, r 42–80) with PTCL [angioimmunoblastic (n=6), PTCL,nos (n=8), extranasal NK (n=2), Sezary syndrome (n=5)] were prospectively accrued. Fifteen pts (71%) had stage IV disease, nine (43%) had >1 extranodal site, 71% had abnormal LDH, 29% had ECOG PS >1, 43% B-symptoms. A total of 105 courses were delivered (median 6, r 2–6) with only three patients receiving less than 4 courses due to disease progression (n=2) and early death (n=1). This latter event occurred in a 76 yr-old man succumbing to septic shock after the 2nd cycle of treatment. In two elderly patients Ifo was omitted from the 4th course onward due to signs of encephalopathy. Grade 4 thrombocytopenia occurred in 8 pts (38%), grade 4 anemia and grade 3 infection were found in 24% and 33%, respectively. The ORR according to FDG-PET/IWC criteria after the first 3 courses was 86% (95%CI: +/− 15), with 4 partial and 14 complete responses (8CR+6 CRu= 67%; 95%CI: 47–87). Bone marrow tumor clearance after full completion of GIFOX eventually converted 6 CRu in full CR. Among the eight pts eligible for ASCT, 6 had effective CD34+ cells harvest and 4 proceeded to transplant. The 5-year Event-Free Survival was 49%, median survival 30.5 months. For complete responders the probability of relapse was 36.5 % at a median follow-up of 24 months (Figure).
GIFOX retains an attractive therapeutic potential as upfront strategy in PTCL, enabling cytoreduction and SCs mobilization for ASCT consolidation, and allows the safe delivery of a full induction program also to patients aged or unfit for high dose therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal