Abstract
Abstract 2825
Mantle cell lymphoma (MCL) constitutes one of the lymphomas with poorest prognosis at relapse and there are no effective salvage.
The activity shown by the combination Gemcitabine and Oxaliplatin in several types of limfoma, along with its “in vitro” synergistic effect, made this regimen an attractive regimen for salvaging patients with MCL. Against this background, we performed an off-label pilot study in order to assess efficacy and toxicity of the combination R-GemOx in relapsed or refractory patients with MCL.
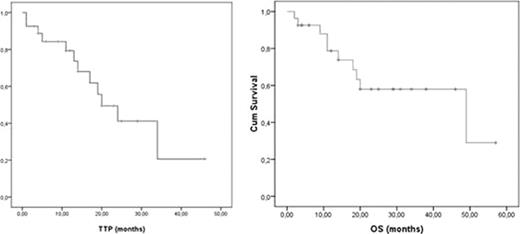
For this, 27 patients (70% male, median age 70 years) diagnosed with MCL between November 2004 and January 2010 were included in this study. Inclusion criteria were adequate performance status, confirmed diagnosis of MCL and relapse or refractoriness to the previous treatment. The regimen consisted of Rituximab 375 mg/m2 on day 1, Gemcitabine 1000 mg/m2 and Oxaliplatin 100 mg/m2 on day 2, every 14 days, up to 8 cycles. Dose and interval were adjusted according to hematological and extrahematological toxicities. Median number of previous regimens was 1 (range 1 to 3), being EPOCH-R (n=14), R-CHOP (n=6), and RFC (n=3) the most frequently used induction therapies. Twelve patients relapsed after prior CR, 10 progressed after achieving a PR, whereas 5 were refractory to therapy. At inclusion, 85% of patients were in advanced (III/IV) clinical stage, 40% had bone marrow infiltration, 28% gastrointestinal involvement, and 17% cavum infiltration. Median number of cycles administered was 8 (range, 3 to 8). Doses were reduced in 9 cycles and delayed in 15 cycles. Neutropenia grade 3–4 was observed in 9 cycles and thrombocytopenia grade 3–4 in 6. Hepatotoxicity grade 1–2 in 5 pts and grade 3 in 1, sensitive neurotoxicity grade 1–2 in 12 pts, and renal impairment grade 2 in 1 patient. After completion of the treatment, 21 pts (77%) were considered in CR/uCR, 1 (4%) achieved a PR, 1 a SD, whereas 4 PD. Six patients subsequently received a stem-cell transplantation (4 allogeneic, 2 autologous). Thirteen pts are still alive and out of progression. Ten pts have died (8 due to progression and 2 due to acute GVHD). With a median follow-up of 23 months (range: 3–57), PFS and OS at 2 years are 41% and 58%, respectively. The R-GemOx combination showed a significant activity in relapsed or refractory pts with MCL with a very acceptable toxicity profile. These results prompted us to conduct a multicenter phase II clinical trial that is now ongoing.
López:Roche Farma: Research Funding, Travel Support. Bosch:Roche Farma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal