Abstract
Abstract 2820
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL), yet there are no standard guidelines regarding the best chemotherapeutic regimens for its management. The most prevalent regimens, especially in advanced disease, incorporate anthracyclines. There is no proof, however, that they are superior to non-anthracycline containing regimens, or even to single agent therapy.
Systematic review and meta-analysis of randomized controlled trials comparing anthracycline-containing regimens (ACR) to non-anthracycline containing chemotherapy (non-ACR) for adult patients with FL. Trials assessing rituximab or other immunotherapy were included only if the anthracycline was the only difference between study arms. A comprehensive search was conducted with no restrictions, until July 2010. Two reviewers appraised the quality of trials and extracted data. The primary outcome was overall survival. Time to event data was extracted and pooled hazard ratios (HR) with 95% confidence intervals (CI) were calculated. Secondary outcomes included survival at 5 years, disease control defined as progression free survival (PFS) or response duration (RD), transformation rate to aggressive lymphoma, complete remission (CR), overall response (OR), and adverse events. Risk ratios (RR) for dichotomous outcomes were pooled using random effects model. Heterogeneity was assessed using the I2 measure of inconsistency.
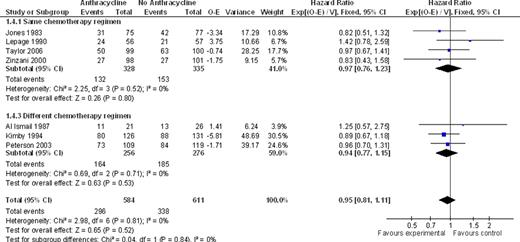
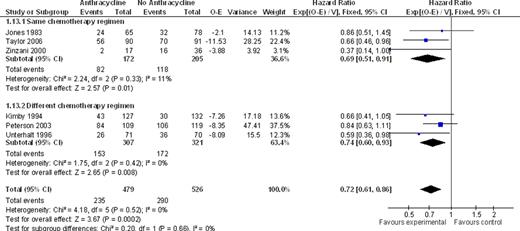
We identified ten trials, conducted between the years 1974–2006 and randomizing 2422 adult patients. Nine trials included naïve patients; one included relapsed and refractory FL patients. Six trials compared between the same chemotherapeutic regimens, with the addition of anthracyclines in the intervention arm as the only difference. There was no statistically significant difference between ACR and non-ACR arms, regarding overall survival at the end of follow up or all cause mortality at five years (HR 0.97; 95% CI 0.76 – 1.23 and RR 0.94; 95% CI 0.76 – 1.17, respectively, I2=0% for both analyses). PFS and RD favored the ACR arm, with a HR of 0.69, 95% CI 0.51–0.91 for disease control, 3 studies, I2=11%. There was no significant difference with regard to CR and OR, but analyses were heterogeneous. In four out of the ten trials, different chemotherapeutic regimens were compared, and only one study-arm contained anthracyclines. Similar to the results obtained with the same regimens comparison, there was no benefit for ACR with regard to OS (HR 0.94; 95% CI 0.77 – 1.15) and mortality at five years (RR 0.98; 95% CI 0.84 – 1.16). Disease control was improved with ACR (HR 0.74; 95% CI 0.60–0.93). In all studies, there were no data regarding transformation rate to aggressive lymphoma. Toxicity data reported was insufficient for appropriate meta-analysis. Overall, ACR were more often associated with cytopenias, especially neutropenia, although the frequency of serious infections or death related to chemotherapy was similar to non-ACR. Also, alopecia and gastrointestinal side-effects, including nausea and vomiting, were more common with ACR. Cardiotoxicity was reported in five trials, and albeit rare, was associated with anthracycline use (RR 8.62; 95% CI 1.58 to 47.05).
The use of anthracyclines in patients with FL has no demonstrable benefit on overall survival. However, ACR improve disease control, as measured by progression free survival and response duration with an increased risk for side effects, notably cardiotoxicity.
Anthracycline vs. no anthracycline for OS
Anthracycline vs. no anthracycline for disease control
Shpilberg:Roche: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal