Abstract
Abstract 2797
Chemotherapy employing the RCHOP every 21 day regimen has become the standard of care for patients with DLBCL. Although the GELA study did include older patients, NHL incidence rises more steeply with age, with a third of cases occurring in patients over 75 years of age. Community oncologists see an ever-increasing NHL age group with multiple co-morbidities. There is also debate whether DLBCL is more aggressive/chemo-resistant at the higher age range. For many, cardiac issues exclude them from an adriamycin-based regimen and possible cure. Since pegylated liposomal doxorubicin (PLD) has a reported lower cardiac toxicity profile even at higher overall doses than doxorubicin, it was substituted into a dose-dense RCHOP regimen. To this end, we developed a phase II dose-dense/dose escalation study of PLD (Doxil®,20 mg/m2: cohort I, 25 mg/m2: cohort II, 30 mg/m2: cohort III), cyclophosphamide (CY, 750 mg/m2), vincristine (1.4 mg/m2: 2 mg max), prednisone (100 mg PO days 1–5) and rituximab (375 mg/m2) on day 1 followed by pegfilgrastim (Neulasta®, 6 mg, SC) on day 2 of a 14 day cycle. Patients received a planned 6 cycles of chemotherapy or 2 cycles past best response. Patients >60 years or with left ventricular ejection fractions (LVEF) <45% were enrolled with ECOG performance status (PS) of 0 – 3. Three, 6 and 8 patients (17 total) were enrolled on cohorts I – III, respectively, Intent to treat (ITT) data included all patients. Response/survival data excluded 2 patients who deteriorated by start of cycle 1 chemotherapy. Safety and SAE's were assessed with each cohort. Quantitative LVEF was obtained with each cycle, CT's every 3 cycles and PET/CT at baseline and within 60 days of chemotherapy completion. Median age was 78 (62-87), 59% were female and baseline LVEF from 12% (with AICD but ECOG PS1) to 87% (median 60%). There was no reduction in LVEF for patients receiving >1 cycle of chemotherapy. Patients who were hospitalized (PS>2) or who's PS declined rapidly between study entry and cycle 1 initiation rapidly became too moribund to complete planned therapy. Relative dose intensity [RDI: (delivered chemotherapy/time to complete)/(planned chemotherapy/planned time to complete)] for the entire group averaged 96 (83 – 100)% for PLD, median 100%, and 97 (86 – 100)%, median 100%, for cyclophosphamide. Nearly all patients (92%) achieved a CR/nCR with a 77% CR rate. Despite an every 14 day anthracycline regimen, Grade >2 hematologic toxicities were manageable and others were low.
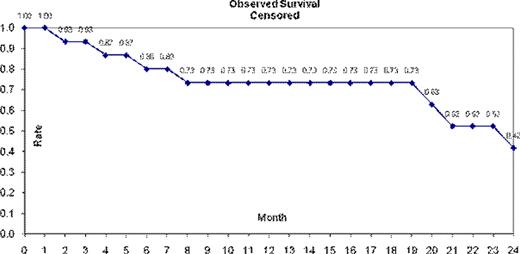
Overall Survival in the ITT population was 65% and 37% at 12 and 24 months, respectively. Censoring for patients removed in or after cycle 1 yielded a survival rate of 73% at 12 months and 42% at 24 months. This elderly patient population had significant long term morbidities, post-chemotherapy, leading to mortality from cardiac disease, ARF and liver failure besides lymphoma related causes. Adjusting for the non-lymphoma deaths gave adjusted survivals of 85% and 71% at 12 and 24 months, respectively. We conclude that it is feasible to deliver a dose-dense anthracycline regimen to geriatric patients with acceptable toxicity. Indeed, 71% of study patients were ≥ 70 years and 47% were ≥ 80 years. Microarray analysis may pinpoint which elderly patients may require a more intensive regimen to effect cure.
| Grade (%) . | N . | F/N . | Hosp F/N . | Tpenia . | PPE . | Cardiac . | Stomatitis . |
|---|---|---|---|---|---|---|---|
| All | 88 | 24 | 24 | 82 | 65 | 24 | 47 |
| 3 | 24 | 12 | 12 | 24 | 6 | 12 | 6 |
| 4 | 41 | 12 | 12 | 6 | 6 | - | - |
| Grade (%) . | N . | F/N . | Hosp F/N . | Tpenia . | PPE . | Cardiac . | Stomatitis . |
|---|---|---|---|---|---|---|---|
| All | 88 | 24 | 24 | 82 | 65 | 24 | 47 |
| 3 | 24 | 12 | 12 | 24 | 6 | 12 | 6 |
| 4 | 41 | 12 | 12 | 6 | 6 | - | - |
Neutropenia =N,
Febrile Neutropenia =F/N,
Hospitalization for F/N = Hosp F/N,
Thrombocytopenia = Tpenia,
Palmar- Plantar erythrodysesthesia = PPE
Noga:Amgen: Honoraria, Research Funding, Speakers Bureau, none; Millenium Takada: Consultancy, Honoraria, Research Funding, Speakers Bureau, none; Ortho-Centicor: Research Funding, Speakers Bureau, none; Cephalon: Honoraria, Speakers Bureau, none; Pfizer: Speakers Bureau, none; Cellgene: Honoraria, Research Funding, Speakers Bureau, none. Off Label Use: Doxil (pegylated liposomal doxorubicin) in place of adriamycin in a CHOP-R regimen for DLBCL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal