Abstract
Abstract 262
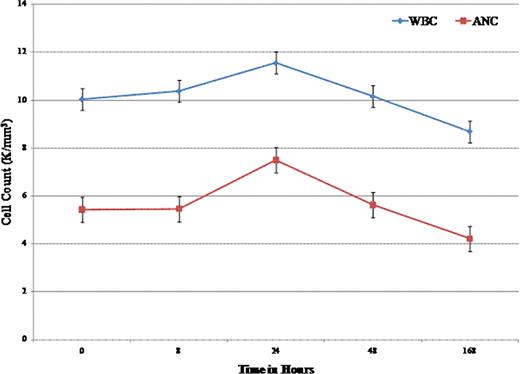
Adhesion molecules are critically involved in the pathophysiology of sickle cell disease (SCD). A growing body of evidence from animal models and humans supports the role of selectin-mediated cell adhesion in the pathophysiology of vaso-occlusion. Leukocyte adhesion has been demonstrated to reduce microvascular blood flow in a sickle cell mouse model, and there is evidence to suggest leukocyte adhesion in humans contributes to vaso-occlusion (Stuart et al, Lancet 2004; Turhan et al, PNAS 2002). GMI-1070 is a pan-selectin inhibitor that targets E-, P-, and L-selectins and has previously been shown to restore blood flow and improve survival in a mouse model of vaso-occlusion (Chang et al, Blood 2010). Also, it is potent inhibitor of adhesion of human neutrophils to immobilized E-selectin and ICAM-1 under flow conditions in vitro. As part of a Phase 1/2 study of GMI-1070 in patients with SCD we determined the effects of the drug on biomarkers of adhesion in vivo and ex vivo. Methods: An open-label phase 1/2 study of the safety, PK, and activity of GMI-1070 was performed, enrolling adults with SCD at steady state. Here we are reporting data on GMI-1070 anti-adhesion activity. GMI-1070 was administered in two IV doses given on the same day: 20 mg/kg as a loading dose, followed 10 hours later by 10 mg/kg. Serial WBC count with differential (all 15 subjects), computer-assisted intravital microscopy (CAIM) (4 subjects), and ex vivo activity of GMI-1070 in plasma (4 subjects) were measured. CAIM is a non-invasive technique for quantitative measurement of microvascular blood flow in vessels of the bulbar conjunctiva. In this study it was used to measure RBC velocity before and after dosing (at 30 minutes, 2, 4, 8, and 24 hours) with GMI-1070. Ex vivo evaluation of plasma GMI-1070 activity in a cell adhesion assay was also performed, with samples taken at 0, 8, 24, and 48 hours after first infusion. Results: Adults were enrolled at three centers: 13 with HbSS, 2 with HbSB0thal. All were African-American, and 9 were male. All subjects received both doses of study drug. The t1/2 was 7.7 hours. Mean baseline WBC was 10 K/mm3, and baseline absolute neutrophil count (ANC) was 5.4 K/mm3. The mean WBC was 10.4, 11.6, 10.2, and 8.7 K/mm3 at 8, 24, 48 hours and 7 days, respectively. The ANC mean was 5.5, 7.5, 5.6, and 4.2 K/mm3 at the same time points. ANC % change from baseline was significant at 24 and 48 hours (p=0.001, 0.025 mixed effects model) (Figure). There were no significant changes in absolute monocyte or lymphocyte counts. There was no correlation with any clinical adverse events. In one subject, the WBC rose from 10.4 to a peak of 28, with no clinical symptoms or significant changes in other lab values. CAIM (n=4) evaluating microvascular blood flow in the bulbar conjunctiva (measured in screen pixels/sec), showed mean RBC velocity at baseline was 335 (SD 70) pixels/sec, with mean values at 30 min., 2, 4, and 8 hours of 368 (59), 345 (63), 341 (59), and 338 (83) pixels/sec respectively, and returned to baseline of 317 (82) pixels/sec at 24 h. These differences did not reach statistical significance (mixed effects model). In ex vivo evaluation of neutrophil adhesion to matrix proteins (as quantified by # of bound neutrophils per 50× field) from samples 0, 4, 8, 24, and 48 hours after the first infusion revealed reduction in adhesions at 4 and 8 hours; mean adhered neutrophils were 33 (17), 20 (11), 18 (9), 47 (49), and 42 (15) respectively. However, these differences did not reach statistical significance. In conclusion, GMI-1070 infusion resulted in neutrophilia, a trend towards increased RBC velocity in the vessels of the bulbar conjunctiva immediately after infusion, and reduced leukoctye adhesion in an ex vivo assay despite neutrophilia. All of these findings are consistent with an anti-adhesive effect on leukocyte adhesion in vivo and suggest that the findings in sickle mouse studies can be translated into SCD patients. This study supports further evaluation of GMI-1070 for the treatment of vaso-occlusive episodes in SCD.
Disclosures:
Wun:GlycoMimetics: Clinical Trial Sponsorship, Consultancy; Eli Lilly: Clinical Trial Sponsorship, Consultancy. Off Label Use: This drug (GMI-1070) has not been approved for any clinical indication. De Castro:GlycoMimetics: clinical trial sponsorship. Styles:GlycoMimetics: Clinical Trial Sponsorship, Consultancy. Cheung:GlycoMimetics: clinical trial sponsorship. Chase:GlycoMimetics: clinical trial sponsorship. Simon:GlycoMimetics: Research Funding. Magnani:GlycoMimetics: Employment, Equity Ownership. Thackray:GlycoMimetics: Employment, Equity Ownership.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2010 by The American Society of Hematology
2010

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal