Abstract
Abstract 2540
Hematopoietic stem cell transplantation (HSCT) from HLA haplo-identical family donors is promising as a therapy for patients with leukemia who are at high risk for relapse. The lower relapse rates and improved survival, especially for patients with acute myelogenous leukemia (AML) who have received HSCT from killer cell immunoglobulin-like receptor (KIR) ligand-mismatched donors, suggest that donor NK cells that are alloreactive against the recipient's cells cause graft-versus-leukemia effects. An Italian group and we identified genomic loss of the patient-specific HLA haplotype in leukemic cells after haplo-identical HSCT. Analysis using SNP arrays revealed that the HLA loss in 29 to 66% of relapsed patients after haplo-identical HSCT was caused by segmental uniparental disomy (UPD) of the HLA region on chromosome 6. This suggested that leukemic cells often escape immunosurveillance through the loss of the mismatched HLA haplotype via the UDP mechanism after haplo-identical HSCT (Vago et al. N Engl J Med. 2009, Villalobos, IB et al. Blood 2010). Since NK cell effector function is tightly regulated by inhibitory KIRs on NK cells that bind to MHC class I on target cells, the escape of leukemic cells from immune surveillance by losing a mismatched HLA antigen might enhance the cytotoxicity of NK cells towards target cells. We examined alterations in donor-derived alloreactive NK cell activity against leukemic blasts of AML patients who relapsed after HLA haplo-identical HSCT.
We enrolled three patients with AML, aged 2, 3 and 12 years, who relapsed after HLA haplo-identical HSCT with T cell depletion of rabbit ATG in vivo. Two patients had AML M7 and the other had M0. Only one donor was KIR ligand-mismatched. Engraftment was achieved in all three patients within 28 days. Relapse occurred 35, 372 and 445 days after HSCT. Mononuclear cells were obtained from both donors and patients before and after HSCT. The NK cells were purified using NK cell selection kits (DYNAL) and measured by conventional 51Cr release assays of leukemic blasts from the patients and the control cell line K562. The mismatched HLA expression between patients and donors on hematopoietic cells was monitored by flow cytometry using anti-HLA antibody (One Lambda).
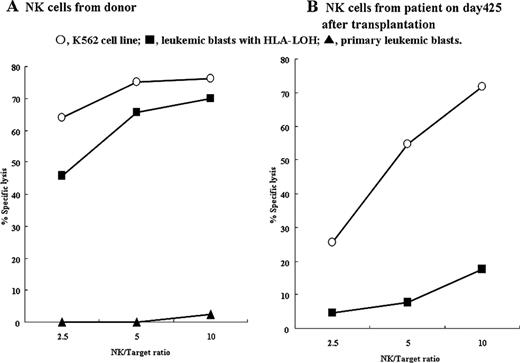
The mean of NK cell killing of the patients' leukemic cells at the same effector target ratio of 10 was significantly higher in donors (50.3%) than in relapsed patients (9.7%). Monitoring of alloreactive NK cell activity after HSCT showed that donor-derived NK cell killing against the patients' leukemic cells gradually decreased in the KIR ligand-mismatched setting. Relapse was minimal but obvious according to HLA monitoring of hematopoietic cells on day 98, which was 7 days after alloreactive NK cell activity was diminished in the patients. Monitoring HLA expression after HSCT also revealed that one patient had leukemic blasts at relapse with loss of the patient's specific HLA haplotype caused by UDP of the HLA region on chromosome 6. Notably, primary leukemic blasts in this patient were not killed by the donor NK cells (2.3%), but leukemic blasts at relapse were efficiently killed (69.0%) after HLA loss of leukemic cells. On the other hand, killing of leukemic blasts at relapse by the patient's NK cells after transplantation was much less effective (19.0%) than that by donor NK cells, although they originated from the same donor (Figure). Because haplotype loss of HLA caused by UDP does not change the status of the KIR ligand in patients with homozygous HLA-Cw, we further examined the expression of ULBP1-3 that are ligands for the activating NK receptor, NKG2D, on leukemic blasts before and after HLA loss. We found upregulated ULBP-2 expression on leukemic cells after the loss of HLA.
Donor NK cells efficiently killed patients' AML blasts at relapse but NK cell activity in patients against their own leukemic blasts was impaired after HSCT. These findings indicate a rationale for donor NK cell infusions after HLA haplo-identical HSCT to avoid decreasing NK cell alloreactivity and to prevent the escape of leukemic cells from allo-immune surveillance by donor cytotoxic T lymphocytes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal