Abstract
Abstract 2383
Allogeneic stem cell transplantation (SCT) is the only potentially curative therapy for patients with CLL. We reported that a major barrier to patients receiving SCT is an inability to attain the necessary disease control with available salvage regimens to proceed to transplant, emphasizing the need to continue to look for alternative therapies. Flavopiridol, a cyclin-dependent kinase inhibitor that induces apoptosis independently of p53, is one such alternative. We sought to determine the outcomes of transplant in patients who had received flavopiridol. Patients and methods: Patients included in this evaluation were enrolled on OSU 0055, 0491, 0211, or 06124 and went on to receive allogeneic SCT. Survival curves were performed using the Kaplan-Meier method. PFS and OS were calculated from the date of the transplant.
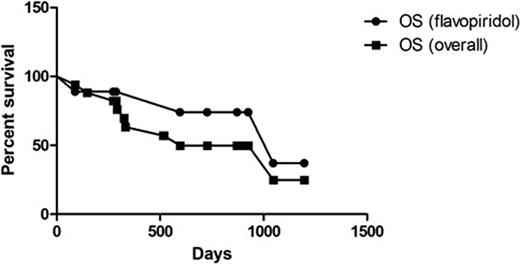
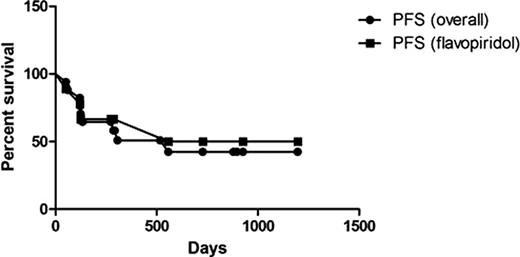
Of the 50 patients who underwent reduced-intensity conditioning (RIC) allogeneic SCT at Ohio State between January 1, 2002 and June 29, 2010, 19 received flavopiridol prior to transplant; nine responded and proceeded directly to transplant. Ten patients received further treatment after flavopiridol: 6 patients had progressive disease, 3 were removed from protocol, and 1 had a prolonged response. Pre-transplant characteristics are summarized in Table 1. Notably, 78% of responders had del(17p13.1), and 33% had bulky lymphadenopathy prior to treatment with flavopiridol. Patients received a median 5 lines of treatment (range 2–11) prior to transplant. Transplant results are summarized in Table 2. After a median observation period of 17.2 months following transplant (range 0.2–39.9), 10 patients were still alive, 5 of whom responded to flavopiridol. Four deaths were due to complications of the transplant, and 3 were due to disease progression. The median time to ANC recovery > 500 was 16 days. Seventeen patients were evaluable for response. Seven patients (41%) had CR following transplant, 6 of whom were MRD-negative. Four of the MRD-negative patients in CR had responded to flavopiridol. Acute GVHD was primarily grade 2 or less, and 50% of patients did not develop chronic GVHD. KM estimates of overall and progression-free survival are included in Figures 1 and 2. Estimated median OS overall is 19.8 months with PFS of 18.6 months; the median OS for the subset of patients who responded to flavopiridol was 34.9 months with median PFS of 29.2 months.
Pre-transplant characteristics
| . | All patients (percent) n=19 . |
|---|---|
| Median age | 60 |
| (range) | 38–74 |
| Sex | |
| male | 13 (68) |
| female | 6 (37) |
| Median KPS | 90 |
| Disease status | |
| CR | 1 (5) |
| PR | 9 (47) |
| SD | 1 (5) |
| Rel | 1 (1) |
| Ref | 7 (37) |
| Cytogenetics | |
| 13q | 3 (16) |
| normal | 0 |
| trisomy 12 | 1 (5) |
| 11q | 2 (11) |
| 17p | 13 (68) |
| Complex? | |
| no | 8 (42) |
| yes | 11 (58) |
| Median # tx | 5 |
| range | 2–11 |
| Donor source | |
| HLA-matched sib | 5 (28) |
| HLA-mm sib | 0 |
| HLA-matched unrel | 10 (56) |
| HLA-mm unrel | 3 (17) |
| Median CD34 dose (million) | 4.04 |
| Median CD3 dose (million) | 2.21 |
| Conditioning regimen | |
| FluBu | 1 (6) |
| FluBuATG | 14 (78) |
| CamFluTBI | 3 (17) |
| GVHD prophylaxis | |
| FKMTX | 15 (79) |
| FKMMF | 4 (21) |
| . | All patients (percent) n=19 . |
|---|---|
| Median age | 60 |
| (range) | 38–74 |
| Sex | |
| male | 13 (68) |
| female | 6 (37) |
| Median KPS | 90 |
| Disease status | |
| CR | 1 (5) |
| PR | 9 (47) |
| SD | 1 (5) |
| Rel | 1 (1) |
| Ref | 7 (37) |
| Cytogenetics | |
| 13q | 3 (16) |
| normal | 0 |
| trisomy 12 | 1 (5) |
| 11q | 2 (11) |
| 17p | 13 (68) |
| Complex? | |
| no | 8 (42) |
| yes | 11 (58) |
| Median # tx | 5 |
| range | 2–11 |
| Donor source | |
| HLA-matched sib | 5 (28) |
| HLA-mm sib | 0 |
| HLA-matched unrel | 10 (56) |
| HLA-mm unrel | 3 (17) |
| Median CD34 dose (million) | 4.04 |
| Median CD3 dose (million) | 2.21 |
| Conditioning regimen | |
| FluBu | 1 (6) |
| FluBuATG | 14 (78) |
| CamFluTBI | 3 (17) |
| GVHD prophylaxis | |
| FKMTX | 15 (79) |
| FKMMF | 4 (21) |
Engraftment Characteristics
| . | All patients (percent) . | Responders (percent) . |
|---|---|---|
| n=17 | n=9 | |
| TRM? | ||
| yes | 4 (24) | 3 (33) |
| no | 5 (29) | 1 (11) |
| ANC <500 | ||
| 10-15 days | 7 (41) | 3 (33) |
| 16-20 days | 5 (29) | 4 (44) |
| >20 days | 5 (29) | 2 (22) |
| Plt <20 | ||
| never | 8 (47) | 5 (56) |
| 10-15 days | 3 (18) | 2 (22) |
| 16-20 days | 4 (24) | 1 (11) |
| >20 days | 2 (12) | 1 (11) |
| aGVHD max | ||
| None | 4 (24) | 1 (11) |
| Grade 1-2 | 11 (65) | 7 (78) |
| Grade 3-4 | 2 (12) | 1 (11) |
| cGVHD max (n=8) | ||
| None | 8 (47) | 4 (50) |
| Limited | 5 (29) | 3 (38) |
| Extensive | 4 (24) | 1 (13) |
| . | All patients (percent) . | Responders (percent) . |
|---|---|---|
| n=17 | n=9 | |
| TRM? | ||
| yes | 4 (24) | 3 (33) |
| no | 5 (29) | 1 (11) |
| ANC <500 | ||
| 10-15 days | 7 (41) | 3 (33) |
| 16-20 days | 5 (29) | 4 (44) |
| >20 days | 5 (29) | 2 (22) |
| Plt <20 | ||
| never | 8 (47) | 5 (56) |
| 10-15 days | 3 (18) | 2 (22) |
| 16-20 days | 4 (24) | 1 (11) |
| >20 days | 2 (12) | 1 (11) |
| aGVHD max | ||
| None | 4 (24) | 1 (11) |
| Grade 1-2 | 11 (65) | 7 (78) |
| Grade 3-4 | 2 (12) | 1 (11) |
| cGVHD max (n=8) | ||
| None | 8 (47) | 4 (50) |
| Limited | 5 (29) | 3 (38) |
| Extensive | 4 (24) | 1 (13) |
Flavopiridol was effective for inducing a disease response in this group of heavily pretreated patients, the majority of whom had del(17p13.1), the sole cytogenetic indication for transplant in first remission in CLL. The patients who responded to flavopiridol and could proceed directly to transplant had longer OS and PFS, although this was not statistically significant. Furthermore, we have observed that flavopiridol is associated with low occurrence of infection when compared with available salvage regimens and thus represents an effective strategy to bridge patients to transplant in a timely manner.
Jones: GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbott Laboratories: Research Funding. Lin: GlaxoSmithKline: Consultancy, Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal