Abstract
Abstract 2362
HLA-Haploidentical Bone Marrow Transplantation (BMT) for High Risk Hematologic Malignancies Using Myeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide
Heather J Symons, Allen R. Chen, M. Sue Leffell, Marianna Zahurak, Kathy Schultz, Heather Karaszkiewicz, Leo Luznik, Javier Bolanos-Meade, Yvette Kasamon, Lode Swinnen, Douglas Gladstone, Richard J. Jones, Ephraim J. Fuchs
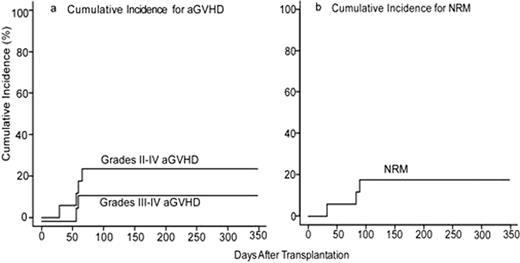
Historically, myeloablative BMT using T cell-replete bone marrow from partially HLA-mismatched (HLA-haploidentical) related donors has been associated with excessive rates of severe graft-versus-host disease (GVHD) and non-relapse mortality (NRM). Based upon promising clinical outcomes using high dose, post-transplantation cyclophosphamide (Cy) as GVHD prophylaxis after non-myeloablative, HLA-haploidentical BMT(Luznik, BBMT 2008;14:641) or after myeloablative, HLA-matched BMT (Luznik, Blood 2010;115(16):3224), we evaluated the safety and efficacy of high-dose, posttransplantation Cy after myeloablative conditioning and T cell-replete, HLA-haploidentical BMT for advanced and refractory hematologic malignancies. Seventeen patients (median age 40, range 13–64; 7 AML, 1 bilineage leukemia, 2 CML, 6 NHL, 1 grey zone lymphoma) have been enrolled, the majority (88%) of whom were not in remission at the time of transplant. Conditioning consisted of IV Busulfan (pharmacokinetically adjusted) on days -6 to -3, Cy (50mg/kg/day) on days -2 and -1 in sixteen patients or Cy (50mg/kg/day) on days -5 and -4 and total body irradiation (300cGy /day) on days -3 to 0 in one patient, followed by T-cell replete bone marrow in all patients. Postgrafting immunosuppression consisted of Cy (50 mg/kg/day) on days 3 and 4, mycophenolate mofetil for 35 days, and tacrolimus for 6 months in all patients. Donor T-cell engraftment occurred in all evaluable patients (n=13). The median times to neutrophil (>500/μ L) and platelet recovery (>20,000/μ L) are 24 days (range, 17–44 days) and 22 days (range, 13–91 days), respectively. On competing risk analysis, the cumulative incidences of grades II-IV and grades III-IV acute graft versus host disease (aGVHD) at day 100 are 24% (95% CI: 7%, 46%) and 12.5% (95% CI: 1.8, 33.9%), respectively (Figure a). On competing risk analysis, the cumulative incidence of chronic GVHD (cGVHD) at one year is 14.7% (95% CI: 0.76, 47.1%). On competing risk analysis, the cumulative incidence of NRM at 100 days is 18% (95% CI: 4%, 39%) (Figure b). NRMs were due to multi-organ system failure in two patients with bulky mediastinal lymphoma and veno-occlusive disease in one patient who had received gemtuzumab ozogamicin 3 weeks prior to BMT. There have been no infectious deaths to date. On competing risk analysis, the cumulative incidence of relapse at 100 days is 29% (95% CI: 10%, 52%) in this poor-risk cohort. With a median follow-up of 10.1 months (range 5.3–12.8 months) in those without events, actuarial overall survival (OS) is 58% at 6 months and 37% at one year and actuarial event-free survival (EFS) is 22% at 6 months and 14% at one year. Myeloablative HLA-haploidentical BMT with T cell replete bone marrow and posttransplantation Cy is associated with promising rates of engraftment, GVHD, and NRM that do not appear substantially different than that seen with non-myeloablative haploidentical BMT with posttransplantation Cy or with myeloablative matched BMT. Based on these data, this approach is being moved into better risk patients.
Kasamon: Genentech: Research Funding. Swinnen: Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal