Abstract
Abstract 2320
The therapeutic potential of imatinib in chronic graft-versus-host disease (cGVHD) is thought to derive from abrogation of the agonistic effects of anti-PDGFRA antibodies (APA) previously identified in patients with extensive cGVHD. Here, we update outcomes from a 15 subject phase 1 dose escalation trial of imatinib for steroid dependent cGVHD and provide correlation with APA measurements. We also determine the prevalence of APA in 120 allo-HCT patients and their donors demonstrating that donor and pre transplant plasma sample APA measurements predict for cGVHD.
All trial subjects had extensive steroid dependent cGVHD previously treated with ≥2 agents. Steroid dependence was defined as progressive cGVHD refractory to prednisone ≥0.5 mg/kg/d for ≥ 1 month or cGVHD requiring prednisone ≥0.25 mg/kg/d for ≥ 3 months. Subjects were treated with imatinib for ≥ 6 months starting at 200 mg daily with dose escalation to 400 mg after 4 weeks as tolerated and if a complete response did not occur. Steroids and one other immunosuppressant were allowed. Steroids were tapered as tolerated and other immunosuppressant dosing remained constant. The primary endpoint was safety and tolerability measured by CTCAE v. 3.0 criteria through 6 months. Secondary endpoints were graded according to the Hopkins' scale for cGVHD assessment, change in daily prednisone requirement, and change in symptom burden measured by the Lee cGVHD scale.
APA in pre-imatinib plasma samples were detected by immunoblot with the extracellular PDGFRA domain (56 kDa) and confirmed by ELISA. Subsequently, 120 consecutive allo-HCT patients with available donor, pre- and 1 year post transplant plasma samples were tested for APA by ELISA.
Median age was 46 years (20-68). Mean time to study enrollment from transplant was 5.1 years and from cGVHD diagnosis 4.1 years. The clinical trial completed accrual on 8/4/09. Imatinib was escalated in 12/15 subjects. Grade 2–3 adverse events (AEs) possibly or probably related to imatinib occurred in 8/12 subjects receiving imatinib 400 mg daily; 5 of these subjects were subsequently dose reduced. Grade 2–3 AEs possibly or probably related to imatinib occurred in 4/15 subjects receiving imatinib 200 mg daily; we concluded this dose to be well tolerated.
Subjects were evaluable for clinical responses after 6 months of therapy. Four subjects were non-evaluable: one subject died secondary to H1N1 infection induced respiratory failure, one subject experienced grade 3 vascular thrombosis and was removed from the study, and two subjects withdrew consent before 6 months. Of 11 clinically evaluable subjects, 3 experienced a major response, 4 experienced a minor response, 2 had stable disease, and 2 experienced progressive disease. Median follow-up was 56.6 weeks (19.1-76.1).
We measured APA in order to directly assess PDGFRA as a relevant imatinib target. APA were detected by immunoblotting in 7/11 evaluable subjects: 4 experienced a major or minor response, 2 had stable disease, and 1 experienced progressive disease. APA were not detected in 4/11 evaluable subjects: 2 experienced a major or minor response, 1 had stable disease, and 1 experienced progressive disease.
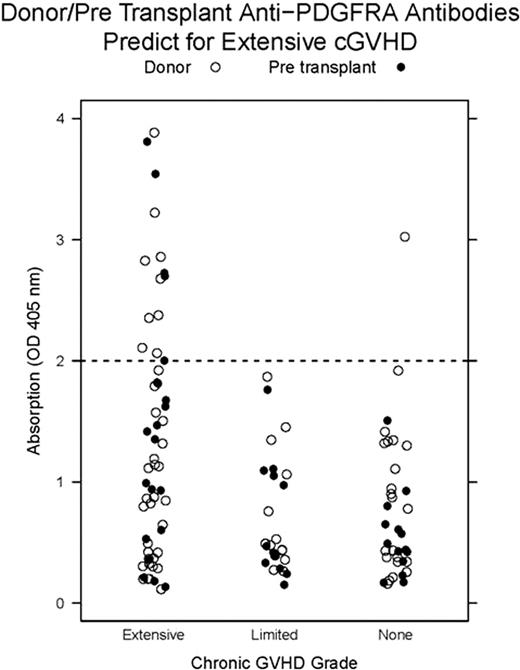
The small sample size precluded interpretation of the significance of APA relative to clinical outcome. Therefore, we extended our APA analysis and measured donor, pre, and 1 year post transplant plasma samples from 120 consecutive allo-HCT patients to determine the clinically relevant APA frequency. Pre transplant and donor APA ELISA measurements ≥ OD 2.0 (absorption at 405 nm) were associated with development of extensive cGVHD (see figure, relative risk=2.23, CI=1.71-2.90). Positive and negative predictive value of this assay for extensive cGVHD was 93% and 58%. Sensitivity and specificity were 24% and 98%. APA measurements in samples 1 year after transplant did not associate with extensive cGVHD (relative risk=0.51, CI=0.09-2.8) explaining the inability of APA to predict response to imatinib in our clinical trial.
1) The response rate of cGVHD to imatinib was 64% warranting further phase 2 studies using 200 mg daily. 2) Pre transplant and donor APA measurements predict for the development of extensive cGVHD. Our APA ELISA may be utilized to improve patient selection in cGVHD prophylaxis trials and to improve donor selection.
Off Label Use: imatinib for cGVHD. Miklos: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal