Abstract
Abstract 2278
BCR-ABL oncogene makes leukemic cells unstable, leading to mutations in BCR-ABL itself as well as other genes1. Studies have shown that prior-to-treatment BCR-ABL mutations do exist in CD34+CD38- (primitive) CML cells2, a population with stem cell like properties responsible for disease persistence, Imatinib (IM) resistance and relapses3. There are also reports regarding association of such pre-existing BCR-ABL mutations (PEM) and Imatinib resistance4. Therefore, the objective of this study was to find BCR-ABL PEM in CD34+38- cells from newly diagnosed CML patients and their correlation with IM resistance.
CD34+CD38- cells from 100 newly diagnosed, untreated, chronic-phase Ph+ CML patients were analyzed for PEM using multiplex allele specific (AS) PCR5 and sequencing AS-PCR products. All of the patients resistant to IM (400mg/day), irrespective of their PEM status, were analyzed by multiplex AS-PCR and DNA sequencing5 for post-therapeutic BCR-ABL mutations, to see if PEM persist in IM resistant patients. T-test was applied to see any significant difference with respect to IM resistance between IM resistant patients with PEM (Group1) and without PEM (Group2).
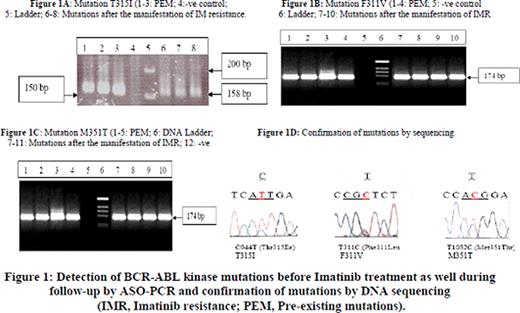
Thirty two out of 100 patients showed at least one PEM (Table 1, Figure 1). During a follow-up of 12–18 months, all patients with PEM manifested IM resistance (32/32,100%). On the other hand, 24/68 patients without PEM manifested IM resistance (35.3%). This shows a significant difference between two groups of patients with respect to IM resistance (100% vs. 35.3%, p=<0.01). On mutational analysis of IM resistant patients, those with PEM persisted with same mutations present before treatment (32/32=100%) while only 21/24 patients manifesting acquired IM resistance (87.5%) harbored BCR-ABL mutations (Table 1). In IM resistant patients, we detected mutations F311V, M351T, Y253F and T315I. All IM resistant patients except with T315I and Y253F showed completed hematological and cytogenetic responses to Imatinib dose escalation (600-800mg/day) or Nilotinib. Our results are in accordance with previous reports3-5. On the basis of this study, it is concluded that BCR-ABL PEM are present in primitive, innately resistant CD34+38- stem cells from a considerable number of CML patients before start of the therapy. This cell population proliferates under selective pressure of the drug to become major cell population, leading to resistant to tyrosine kinase inhibitors like Imatinib and Nilotinib. Therefore, we recommend testing of BCR-ABL mutations in CML stem cell population before start of TKI treatment using sensitive, validated techniques like ASO-PCR and DHPLC, and utilization of this mutation data in clinical management, hence in patient-tailored treatment.
References:
1) Stoklosa T et al. Cancer Res. 2008 Apr 15; 68(8):2576-80
2) Jiang × et al.J Natl Cancer Inst 2007 99: 680–693
3) Chu S et al. Blood (2005) 105:2093–8
4) Jiang × et al. Blood 2010 Jun 23.
5) Kang HY et al. Haematologica 2006 May; 91(5):659-62.
Clinical, cytogenetic and molecular follow-up studies of CML patients with and without prior-to-treatment BCR-ABL mutations receiving Imatinib treatment {%: percentage; PEM: pre-existing mutations; aIR: acquired Imatinib resistance; IM: Imatinib; CHR: Complete hematological response (disappearance of all signs and symptoms of disease); PHR: partial hematological response; CCR: complete cytogenetic response (0% Ph+ cells in bone marrow); PCR: partial cytogenetic response (1-35% Ph+ cells in bone marrow); minor CR: minor cytogenetic response (36-65% Ph+ cells in bone marrow); minimal CR: minimal cytogenetic response (66-99% Ph+ cells in bone marrow); CMR: complete molecular response (BCR-ABL –ve by nested RT-PCR in bone marrow)}(Response criteria as described by Aziz et al, Cancer. 2007 Mar 15;109(6):1138-45.)
| Group . | Category . | Number of patients (%) . | Hematological response (HR) . | Cytogenetic response (CR) . | CMR . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHR . | PHR . | No HR . | CCR . | PCR . | Minor CR . | Minimal CR . | ||||
| Group 1 | Patients with PEM (A) | 32 (100) | 23 (71.8) | 9 (28.2) | – | 17 (43.1) | 7 (21.9%) | 3 (9.4) | 5 (15.6) | – |
| Group 2 | Patients without PEM (B=C/D) | 68 (100) | 62 (91.2) | 3 (4.4) | 3 (4.4) | 38 (55.9) | 19 (27.9) | 5 (7.4) | 6 (8.8) | 28 (41.2) |
| Patients With aIR (C) | 24 (100) | 19 (79.2) | 2 (8.3) | 3(12.5) | 7 (29.2) | 11 (45.8%) | 2 (8.3) | 4 (16.7) | – | |
| IM susceptible Patients (D) | 44 (100) | 43 (97.7) | 1 (2.3) | – | 31 (70.5) | 8 (18.2) | 3 (6.8) | 2 (4.5) | 28 (63.6) | |
| Group . | Category . | Number of patients (%) . | Hematological response (HR) . | Cytogenetic response (CR) . | CMR . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHR . | PHR . | No HR . | CCR . | PCR . | Minor CR . | Minimal CR . | ||||
| Group 1 | Patients with PEM (A) | 32 (100) | 23 (71.8) | 9 (28.2) | – | 17 (43.1) | 7 (21.9%) | 3 (9.4) | 5 (15.6) | – |
| Group 2 | Patients without PEM (B=C/D) | 68 (100) | 62 (91.2) | 3 (4.4) | 3 (4.4) | 38 (55.9) | 19 (27.9) | 5 (7.4) | 6 (8.8) | 28 (41.2) |
| Patients With aIR (C) | 24 (100) | 19 (79.2) | 2 (8.3) | 3(12.5) | 7 (29.2) | 11 (45.8%) | 2 (8.3) | 4 (16.7) | – | |
| IM susceptible Patients (D) | 44 (100) | 43 (97.7) | 1 (2.3) | – | 31 (70.5) | 8 (18.2) | 3 (6.8) | 2 (4.5) | 28 (63.6) | |
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal