Abstract
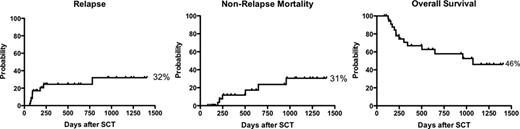
Allogeneic hematopoietic stem cell transplantation (HSCT) strategies must serve as platforms for adoptive cellular immunotherapy if the graft-versus-leukemia (GVL) effect is to be fully exploited. In sequential clinical trials at the National Institutes of Health we have used ex-vivo T cell depletion to develop an HSCT with minimal graft versus host disease (GvHD) prophylaxis followed by elective donor lymphocyte infusion on day 90. T-cell depletion creates the lymphodepleted environment for preferential homeostatic expansion of adoptively infused cells, while minimization of post-transplant immunosuppression promotes expansion of the adoptively transfused cells. Since non-engraftment is a limiting complication with extreme T lymphocyte depletion and reduced GvHD prophylaxis, we measured lineage-specific chimerism and clinical outcomes utilizing our optimized approach. Thirty-six patients with hematologic malignancies underwent allogeneic HSCT with a graft from their HLA-identical siblings. The median age was 43 years (range 16–68), 50% were males. Transplant indications were AML(17), ALL (7), MDS (6), CML (2), CLL (2), NHL (1) and CMMoL (1). 50% were standard risk and 50% were high risk. Subjects received myeloablative conditioning regimen with cyclophosphamide (60 mg/kg/dose × 2), fludarabine (25 mg/m2/dose × 5) and total body irradiation (12 Gy divided in 8 fractions, with lung shielding to 6 Gy). Subjects 55 years of age and older received 4 Gy divided in 8 fractions without lung shielding. G-CSF mobilized peripheral blood stem cells from the donor were CD34+ selected by the Miltenyi CliniMacs system, with infusion of a target CD34+ dose of 6 × 106/kg (range 3 to 10 × 106/kg) and a fixed CD3+ dose of 5 × 104/kg. Low-dose cyclosporine till day 21 was the sole GVHD prophylaxis. Delayed lymphocyte add back (5 × 106 CD3+/kg) was given at day 90 in the absence of significant GvHD. CD3+ and myeloid chimerism analysis were performed sequentially on peripheral blood with early lymphocyte add back in cases with falling chimerism. Day 200 overall survival (the primary study endpoint) was 84%. One patient, who was postpartum, failed to engraft and required a second transplant. 34/36 subjects achieved complete donor (>95%) myeloid chimerism by day 14 and the median time to complete donor CD3+ chimerism was 45 days. The incidence of acute GVHD grade II, III and IV were 23%, 2.9% and 0%, respectively. The incidence of chronic GVHD was 34.3%. At a median follow up of 3.7 years, Kaplan-Meier estimates of relapse, nonrelapse mortality and overall survival were 32%, 31% and 46% respectively. In conclusion, transplants utilizing this approach have acceptable engraftment and clinical outcomes and may serve as an ideal platform for adoptive cellular immunotherapy.
No relevant conflicts of interest to declare.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal