Abstract
Abstract 2071
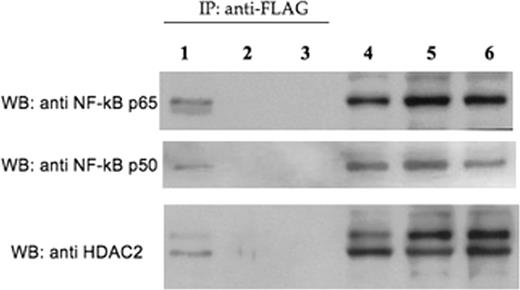
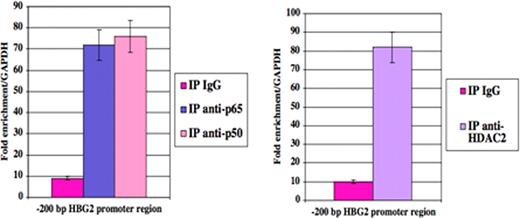
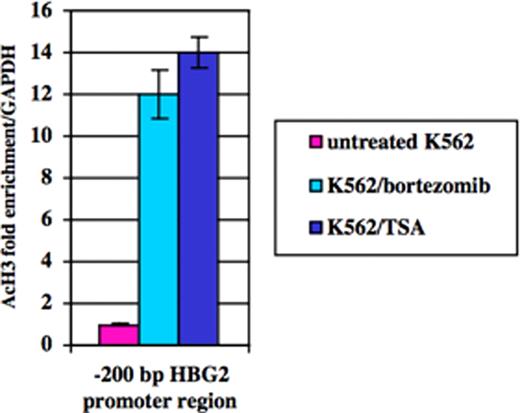
Impaired switching from fetal hemoglobin (HbF) to adult globin gene expression leads to hereditary persistence of fetal hemoglobin (HPFH) in adult life. This is of prime interest because elevated HbF levels ameliorate beta-thalassemia and sickle cell anemia. Fetal hemoglobin levels are regulated by complex mechanisms involving factors linked or not to the beta-globin gene locus. Recently, we reported an inverse relationship between Ggamma-globin gene (HBG2) and Cold Shock Domain Protein A (CSDA) expression levels. Based on mRNA differential display analysis, RNA interference and over-expression studies in K562 and primary erythroid cells we postulated that CSDA could contribute to regulate HBG2 expression. The putative mechanism by which CSDA modulates HBG2 expression was investigated in K562 cells by gene reporter assays on wild-type and mutant constructs of the HBG2 promoter region suspected to bind CSDA, providing experimental evidence that CSDA acts as repressor of HBG2 expression. Furthermore, chromatin immunoprecipitation (ChIP) analysis on K562 cells showed that CSDA interacts in vivo with this promoter region. In this way we were able to demonstrate that CSDA modulates HBG2 expression at least in part at the transcriptional level (Petruzzelli R et al, Br J Haematol 2010). The CSDA gene is located at position 12p13.1 and comprises 10 exons. The C-terminus (exons 6–9) is involved in protein-protein interactions. Alternative splicing of exon 6 results in two main isoforms, namely CSDA isoform a and isoform b, which show different C-terminal domains, potentially able to take part to specific protein complexes. We found that expression levels of CSDA isoform a were reduced in HPFH patients respect to isoform b. These findings suggested that isoform a could be much more involved in repression of HBG2 expression compared to isoform b. To identify putative CSDA interactors, we over-expressed these two FLAG-tagged CSDA isoforms in K562 cells. Western-blot analysis on proteins immunoprecipitated with a FLAG antibody revealed the presence of NF-kB p50 and p65 subunits and histone deacethylase 2 (HDAC2) only in samples co-immunoprecipitated with CSDA isoform a, but not with isoform b (Fig. 1). By ChIP assays with antibodies against p65, p50 and HDAC2, we demonstrated that both the NF-kB p50-p65 heterodimer and HDAC2 interact with the –200 bp region of the HBG2 promoter containing the CSDA binding site (Fig. 2). To examine the role of NF-kB and histone deacetylases on the transcriptional repression of HBG2 expression, we treated K562 cells with the proteasome inhibitor bortezomib which blocks the nuclear traslocation and transcriptional activity of the NF-kB p65-p50 complex or with the histone deacetylase inhibitor trichostatin A (TSA). Quantitative analysis by Real Time PCR showed that HBG2 expression increased following either bortezomib or TSA treatments. Furthermore, by ChIP analysis we were able to demonstrate that knock-down of CSDA abolished these interactions. To investigate if treatment with bortezomib or TSA affects the histone acetylation levels at the -200 bp region of the HBG2 promoter, we performed ChIP assays in K562 cells using an anti-acetyl-H3 antibody. Results indicated that both these drugs induces a considerable increase in H3 acetylation levels at the -200 bp region of the HBG2 promoter (Fig. 3). Taken altogether these data indicate that NF-kB and HDAC2 interact with CSDA to form a multiprotein complex which take part to the regulation of HBG2 expression by modulating local chromatin conformation. Furthermore, our study contributes to better define the role played by CSDA in fetal globin gene expression and shed novel light on the molecular mechanisms involved in globin gene switching.
Western blot analysis with anti-p65, anti-p50 and anti-HDAC2 antibodies on protein extracts from K562 transfected with p3xFLAG-CSDA a (lanes 1 and 4), p3xFLAG-CSDA b (lanes 2 and 5) or p3xFLAG (lanes 3 and 6) immunoprecipitated with an anti-FLAG antibody (lanes 1, 2, 3) or not immunoprecipitated (lanes 4, 5, 6).
Western blot analysis with anti-p65, anti-p50 and anti-HDAC2 antibodies on protein extracts from K562 transfected with p3xFLAG-CSDA a (lanes 1 and 4), p3xFLAG-CSDA b (lanes 2 and 5) or p3xFLAG (lanes 3 and 6) immunoprecipitated with an anti-FLAG antibody (lanes 1, 2, 3) or not immunoprecipitated (lanes 4, 5, 6).
Real-time PCR analysis on ChIP samples from K562 cells showing that NF-kB and HDAC2 interact with the HBG2 proximal promoter region.
Real-time PCR analysis on ChIP samples from K562 cells showing that NF-kB and HDAC2 interact with the HBG2 proximal promoter region.
Quantitative real-time PCR analysis from K562 DNA immunoprecipitated with anti-acetyl-H3 or IgG antibodies following bortezomib or TSA treatments.
Quantitative real-time PCR analysis from K562 DNA immunoprecipitated with anti-acetyl-H3 or IgG antibodies following bortezomib or TSA treatments.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal