Abstract
Abstract 1978
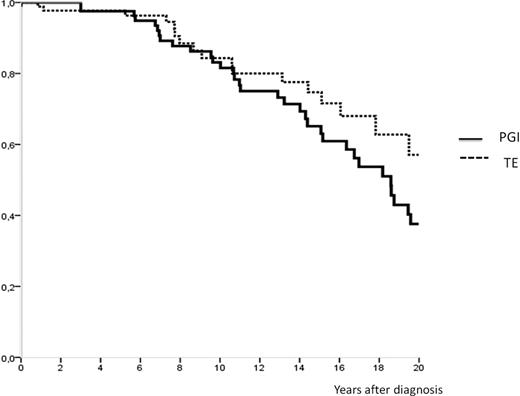
To study the evolution and prognosis in familial cases of myeloproliferative neoplasms (MPN), we collected clinical data and blood samples from 94 families defined by the presence of at least two affected subjects with one MPN. A total of 228 subjects including 99 polycythemia vera (PV), 104 essential thrombocythemia (ET), 14 primary myelofibrosis (PMF) and 11 chronic myeloid leukaemia (CML) were recruited. Occurrence was vertical in 63 families involving 2 generations in 48, 3 generations in 13, and two other families implicating respectively 4 and 5 generations. The distribution of MPN was most compatible with autosomal dominant inheritance. Phenotypic spectrum within families was either homogenous (56/94) or heterogeneous (35/94) and the main association (21/35) was PV and ET. Clinical and haematological data of affected subjects at diagnosis were similar to sporadic MPN. The V617F JAK2 mutation was found in 87% of patients with PV, in 59% of patients with ET, in 64% with PMF and in none patient with CML. 99 PV patients were studied (median age, 54 years; median follow-up, 12 years). Overall survival was 83% at 10 years and 37% at 20 years. At time of analysis 53% were alive; death was due to the hematologic malignancy in 74% of cases. Progression to acute leukemia and secondary myelofibrosis risks were respectively 21% and 20%, after a median time of 14 years (5,4-32,5) and 13 years (4,7-31). No difference in frequency of transformation in acute leukemia was observed between the JAK2 positive (23%) and JAK2 negative (20%) PV patients. Interestingly, none of JAK2-negative PV patients progressed to myelofibrosis, whereas 13 (20%) of JAK2-positive patients suffered from this adverse evolution. A high JAK2 allele burden was correlated with transformation to myelofibrosis. Of 49 patients with < 50% JAK2 mutated allele burden, only 2 (5%) developed myelofibrosis, whereas of 23 patients with ≥ 50% allele burden, 11 (48%) developed this complication (p<0,0001). Thrombosis occurred in 42% of PV patients, arterial in 19 (50%) cases, venous in 16 (42%), and 2 patients suffered both arterial and venous thrombosis. Hemorrhagic manifestations occurred in 2% of cases. Among the 104 ET (median age, 45 years; median follow-up, 8 years), overall survival was 83% at 10 years and 57% at 20 years. At time of analysis 83% were alive, 70% of deaths were related to the MPN. The risks of progression to acute leukemia and myelofibrosis were 10% and 13%, after a median time of 7,4 years (0,8-24) and 16 years (2,5-20,5) respectively. In particular, two non-related ET patients evolved rapidly after diagnosis, developing acute leukemia within the first year. We found no difference in frequency of transformation to acute leukemia or myelofibrosis according to JAK2 status. 32% ET patients suffered from thrombosis, arterial in 18 (60%) of cases and venous in 10(33%). Hemorrhagic manifestations occurred in 3% of cases. In the 14 PMF patients (median age, 53 years; median follow-up, 8 years), overall survival was 46% at 10 years. Transformation risk to acute leukemia was 23% with a median time of 26 months (1-50). Considering the whole population, occurrence of thrombosis was correlated with JAK2 mutational status, with 18% of any thrombosis in the JAK2-negative group and 37% in the JAK2-positive group (p=0.009). Among these, 12 (26%) patients developed deep venous thrombosis such as portal venous thrombosis or Budd-Chiari syndrome. In conclusion, the analysis of these 94 familial myeloproliferative neoplasms cases highlights that the presence of JAK2 mutation is associated with transformation to myelofibrosis for familial PV patients, and with a more frequent occurrence of thrombosis in the entire population. Patients with familial PV have a comparable prognosis to non familial PV. But, in our cohort, there seems to be an excess mortality of familial ET patients when compared to sporadic ET without clear correlation with JAK2 mutational status. Other genetic events involved in familial myeloproliferative neoplasms could explain this phenomenon.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal