Abstract
Abstract 1967
An initial report of MM011 described the MTD and promising activity in the first 62 patients (pts) (Baz R et al, Ann Oncol 2006). Here we report complete outcomes of the entire study cohort (n=77) with over four years since the enrollment of the last patient.
Pts with relapsed or refractory myeloma after at least 2 cycles of previous therapy were treated with lenalidomide at increasing dose levels (5mg – 10mg – 15mg, MTD 10mg) d1-21 every 28 days, liposomal doxorubicin 40mg/m2 d1, dexamethasone 40mg d1-4, and, if no significant neuropathy was present, vincristine 2mg IV for 4–6 cycles (2 cycles beyond best response) followed by lenalidomide maintenance at 10mg d1-21 every 28 days. Prednisone 50 mg every other day was allowed during maintenance, dexamethasone was not. DVT prophylaxis with aspirin 81 mg daily was mandated. All pts were included in PFS and OS analyses. Patient mortality was verified via social security death index as of July 20, 2010. Pts who developed progressive disease after at least one dose of study drug or who had measurable disease and stayed on study for at least 60 days were considered evaluable for response.
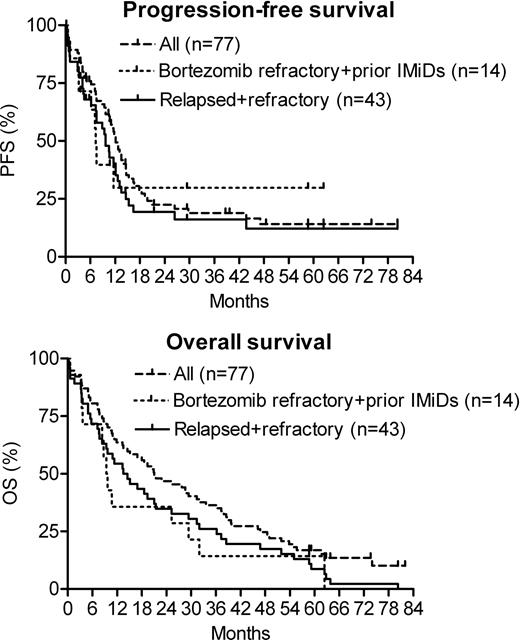
From 03/2003 to 04/2006 77 pts of median age 62 years (range, 41–81) were enrolled, median follow up on study was 16.4 months (range, 0.2–80.4). Median time from diagnosis to enrollment was 39 months (range, 4–118); 67 pts (87%) had Salmon-Durie stage III at diagnosis; median previous therapies were 3 (range 1–7); 49 pts (64%) were refractory to last induction (46 after previous response, 3 primary refractory). Karyotype analysis at study entry revealed abnormal cytogenetics in 28 (36.4%), with del13 in 13 (17.1%) and del17p in 5 pts (6.6%). Previous therapies included doxorubicin (n=60, 78%), thalidomide (n=56, 73%), bortezomib (n=20, 26%), high dose chemotherapy with ASCT (n=17, 22%), and lenalidomide (n=4, 5%). During induction, grade 3 and 4 adverse events occurred in 37 pts (48.1%), with hematologic AEs, VTEs, and infections observed in 32.5%, 6.5%, and 3.9% of pts, respectively. 43 pts (56%) reached maintenance and 41 of them received prednisone in addition to lenalidomide. During maintenance 11 out of 43 pts (25.6%) experienced grade 3 or 4 AEs, (hematologic: 16.3%, infections: 4.7%, VTEs: 4.7%). One patient experienced heart failure that was attributed to severe aortic stenosis. 64 out of 77 pts (83%) were evaluable for response. According to uniform international response criteria with adapted EBMT criteria for MR, their rates of CR, VGPR, PR, and MR were 5%, 16%, 33%, and 20%, respectively, resulting in a 54% response rate (RR=CR+VGPR+PR) and a 74% clinical benefit response rate (CBRR=RR+MR). Median PFS and OS were 12.1 and 21 months, respectively. In the subgroup of 46 pts with relapsed and refractory myeloma, 37 were evaluable for response and achieved a RR of 49% and a CBRR of 65% (VGPR 14%, PR 35%, MR 16%). Median PFS and OS in pts with relapsed and refractory disease (n=46) were 9.7 and 14 months, respectively. Pts with disease relapsed after or refractory to prior thalidomide or lenalidomide and refractory to or relapsing within 60 days after bortezomib (n=14; median age=62.5 years, median previous therapies=4) experienced median PFS and OS of 7.3 and 9.6 months, respectively, as compared to median EFS of 1 and OS of 6 months in a comparable patient population (Kumar S et al, ASH 2009 #2878). Among 12 pts evaluable for response in this highest risk group, RR and CBRR were 25% and 50%, respectively. Their median duration of response was 5.8 months.
DVd-R yielded impressive long-term outcomes in relapsed and refractory myeloma pts, even if their myeloma was refractory to bortezomib.
Baz:Celgene: Research Funding. Hussein:Celgene: Employment. Srkalovic:EPIC: Speakers Bureau; Physicians Connect: Speakers Bureau. Dean:Celgene: Research Funding. Knight:Celgene: Employment. Zeldis:Celgene Corp: Employment. Reu:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal