Abstract
Abstract 183
Acute Myeloid Leukemia - Therapy, excluding Transplantation: Pediatric and Adult AML Therapy
AYAs with acute lymphoblastic leukemia have superior survival when treated with intensive pediatric regimens compared to regimens used for older adults. To determine if a similar benefit occurred in AYAs with AML, we performed a retrospective analysis of 517 patients (pts) with AML ages 16 to 21 years (yrs), comparing outcomes between patients treated on COG (n=281), CALGB (n=149) and SWOG (n=87) studies from 1986 to 2008. We also performed stratified analyses, comparing younger to older patients (16-18 vs 19–21 yrs) and comparing early and later studies. Early studies included those before 1996 (COG: 2861, 2891; CALGB: 8525, 9022, 9222, SWOG 8600). Later studies included COG 2941, 2961 and AML03P1, CALGB 9621, 19808 and SWOG 9500 and S0106.
The median age was 18.0 yrs. However, the COG cohort (17.2 yrs) was significantly younger than CALGB (20.1 yrs) and SWOG (19.8 yrs) (p<0.001); 94% of COG pts were <19 yrs. The median white blood cell count, percent bone marrow blasts, FAB classification and overall complete remission rates did not differ between the Groups. The analysis of cytogenetics is ongoing as we reconcile differences between varying cooperative group classification systems. Assessment of the cumulative incidence of relapse is ongoing as well. Data on molecular abnormalities were incomplete and not analyzed.
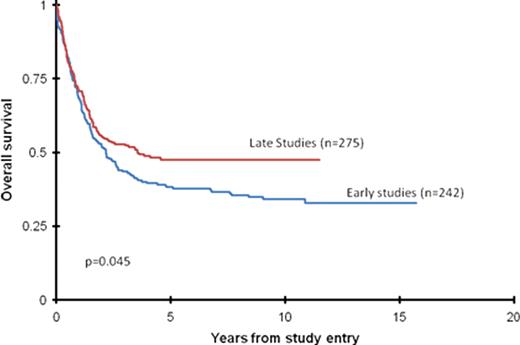
At eight years from the time of study entry, overall survival (OS) was first noted to be significantly higher for the COG cohort than for the combined CALGB/SWOG cohort (45+6% vs. 36+7%) (p<0.001). Combining the patients from the three cooperative groups, the OS in younger AYAs was 43±6% vs 32±8% in older AYAs at 10 yrs (p<0.05). Within each cooperative group, there was no difference in the OS of younger and older AYAs. Combining the patients from the three cooperative groups, the 5 yr OS improved from 38+6% in early studies to 48+6% for AYAs on later studies (p=0.045).
Data on all patients from the 3 cooperative groups showed younger AYAs had a higher 5 yr EFS than older AYAs (37%+5% vs 26%+7% (p=0.015). Younger AYAs in COG had improved EFS compared to older AYAs in COG (39%+6% vs 17%+18%)(p=0.053). There was no significant difference in EFS between younger and older AYAs in CALGB or SWOG, where EFS was 28%+8% vs 27%+10%, respectively. Overall, the 5 year EFS of the COG AYAs (38%+6%) was higher than CALGB and SWOG combined (27%+6%) (p=0.006), although the younger age of the COG cohort is confounding. Combining the data from the 3 cooperative groups, 5 yr EFS has improved from 30+6% in earlier studies to 36+6% in later studies (p=0.039).
One marked distinction between the cooperative groups was the significantly higher treatment-related mortality (TRM) on COG trials. TRM was 29 + 5% at 5 yrs on COG trials compared to 7 + 4% for CALGB and SWOG patients combined (p<0.001). Changes to supportive care guidelines in COG AML trials in 2000 have subsequently led to a decrease in TRM. Combining pts from all 3 cooperative groups, younger AYAs had increased 5 yr TRM of 25+5% compared to 8+4% of older AYAs (p<0.001). However, there were no differences in TRM between younger and older AYAs within each cooperative group, which may be due to the younger age of the COG cohort. Overall, 5 yr TRM has increased from 13+4% on early studies to 24+5% on later studies.
In summary, younger AYAs have improved OS and 5 yr EFS in comparison to older AYAs. Age is a confounding variable in this cohort making it difficult to compare outcomes for AYAs treated on pediatric and adult studies. Even within the small 6-year age span of these AYAs, there are marked differences in the ages of patients treated by pediatric and adult cooperative groups. OS and EFS have increased for AYAs in more recent studies; unfortunately TRM has increased as well.
No relevant conflicts of interest to declare.
This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal