Abstract
Abstract 1793
Elderly patients (pts) with high risk DLBCL are one of the fastest growing demographics of pts, but advances over RCHOP21 have not been realized. These pts are often under-represented in clinical trial secondary to comorbidity, ageism, and eligibility requirements. By the age adjusted international prognostic index (aaIPI), pts >60 years (yrs) with high-intermediate (HI) and high (H) risk disease had 5 yr survival (OS) rates of 37% and 21%. Even in the RCHOP era, high risk elderly patients have event-free-survival of roughly 50%. We report the mature data from a phase II study investigating sequential RCHOP followed by yttrium-90 ibritumomab tiuxetan radioimmunotherapy (RIT) in an effort to improve these results.
Untreated ASCT-ineligible pts >60 yrs, with aaIPI HI and H risk DLBCL were eligible for study. Pts received RCHOP21 × 6 cycles at standard doses with prophylactic GCSF and darbepoetin alfa. Pts with responding (CR/PR) or stable disease post RCHOP received RIT, given 6–9 weeks post chemotherapy at 0.4 mCi/kg for platelets (plt) ≥150K and 0.3 mCi/kg for plt 100–149K. Weekly CBCs were performed for 12–13 weeks or until count resolution. Rituximab levels were assessed pre-RIT in a subset of pts. Primary end-points were PFS and OS, with intent-to-treat (ITT) analysis performed for all patients. Statistics anticipated 2/3 of pts (n=43) receiving RIT, with power to demonstrate a 20% improvement in PFS/OS at 2 yrs.
65 pts were consented, with 2 pts having progression prior to a single cycle on protocol, leaving 63 evaluable pts. The median age was 75 (62-86), median KPS 70% (50-100%), Stage III/IV 23/74%, LDH>ULN 91%, ENS>1=45%, BM positive=11%; aaIPI HI/H 45%/55%. Moderate or greater comorbidity was present in 86% of pts (n=65) by NIA/NCI scale. 86% of pts completed RCHOP, with overall response rate (ORR) of 83% (CR/Cru 75%, PR 8%) (Cheson 1999).
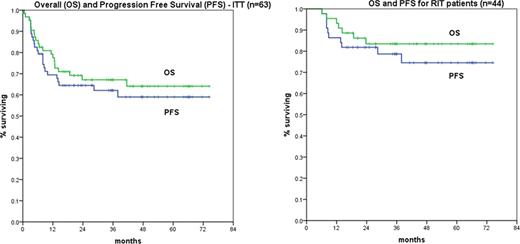
Fifty pts by ITT were eligible for RIT following RCHOP chemotherapy, with 44 pts ultimately treated. Reasons for discrepancy included low bone marrow cellularity (2pt), AFIB/CHF post day 0 (1 pt), abnormal baseline FISH/cytogenetics (2 pts), withdrawal of consent (1pt). Rituximab levels in 30 pts are available pre-RIT: mean 112 mcg/ml (range 41–196) and did not correlate with outcome. The RIT dose was 0.4 mCi/kg in 38 patients and 0.3 mCi/kg in 6 patients. No patient had altered biodistribution on imaging. RIT was generally well tolerated with expected hematologic nadirs at weeks 6–7. Grade 3/4 non-heme toxicity during RIT included neutropenic fever (2), transient EF decline >20% (1), hip fracture (1), anthracycline induced cardiomyopathy (grade 5), suspected CNS bleed (grade 5). Response post RIT (n=44) was CR/CRu 86%, PR 2%. Response improvement (PR->CR or CRu->CR) occurred in 7 pts (16%).
Median followup for surviving pts is 42 months, with median OS and PFS not reached (0.5-74.4). KM estimates of OS and PFS at 42 months by ITT: 64% and 62% respectively. OS and PFS at 42 months for RIT treated pts (n=44): 83.5% and 74.5%. There have been only two relapse >12 months after RIT. The aaIPI (HI vs H) predicted outcomes: OS 75% vs. 49% (p=.008), PFS 68% vs. 45% (p=.01).
Sequential RCHOP21 × 6 cycles followed by RIT in an elderly, high comorbidity, high risk DLBCL cohort is associated with very encouraging outcomes by ITT and specifically in the RIT treated pts with long term follow-up. Therapy was well tolerated in this age group and given lack of late relapses, one may hypothesize RIT is addressing minimal residual disease typically associated with recurrence. Outcome is very favorable when compared to historical controls. A prospective Phase III study is needed to confirm these results.
Hamlin:Genentech: Honoraria, Speakers Bureau; Biogen Idec: Honoraria, Speakers Bureau; Spectrum: Consultancy, Research Funding; CTI: Consultancy, Research Funding. Off Label Use: Y-90 ibritumumab tiuxetan is not approved for use in DLBCL; it is FDA approved for NHL/indolent lymphoma. Rodriguez:CTI: Research Funding. Noy:Spectrum: Consultancy. McLaughlin:Spectrum: Consultancy. Pandit Tasker:CTI: Research Funding; Spectrum: Research Funding. Zelenetz:GSK: Consultancy; CTI: advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal