Abstract
Abstract 1744
Natural Killer (NK) cells are able to extract fragments of cell membrane from antigen presenting cells through the immunological synapse, functionally incorporating the membrane receptors contained therein by a process called trogocytosis. Recently it was demonstrated that NK cells can acquire functional CCR7 from dendritic cells through this process and migrate in response to chemokines (CCL19 and CCL21). We investigated whether this process could be used to transiently modify NK cells ex vivo without genetic intervention.
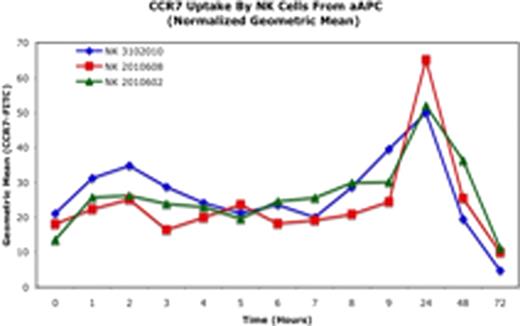
In previous work, we developed a K562-based artificial antigen presenting cell (aAPC) expressing membrane-bound IL-21 (K562-cl9-mIL21) which enables robust NK-cell expansion, and determined that NK cells did not express detectable CCR7 during this process. To investigate trogocytosis in this system, we genetically modified K562-cl9-mIL21 to express membrane-bound CCR7 (K562 cl9 mIL21CCR7) using the Sleeping Beauty transposon/transposase system. After 24 hours of co-culture the NK cells cultured with K562-cl9-mIL21CCR7 demonstrated marked surface expression of CCR7 compared to NK cells cultured on K562-cl9-mIL21 (Figure 1a). In kinetic experiments using three independent donors, CCR7 peak uptake occured at 24 hours (Figure 1b), followed by a decline that corresponded with the loss of aAPCs in the cultures due to lysis by NK cells. This demonstrates that NK cells can be transiently modified during in vitro expansion to bear receptors that are otherwise not a normal part of their transcriptional repertoire. We are currently establishing the functionality of trogocytosed receptors in vitro and in vivo, and investigating the kinetics of CCR7 persistence after removal of the aAPCs. However, even transient expression as demonstrated might be sufficient for bestowing novel NK-cell migration ability in vivo in response to chemokine signaling, giving the engineered NK cells ability to reach desired tissue targets.
Disclosures:
Lee:Altor BioScience Corp: Research Funding; Celgene: Research Funding.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2010 by The American Society of Hematology
2010

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal