Abstract
Abstract 1674
Friend leukemia virus integration 1 (Fli1), a member of the Ets transcription factor family, is expressed in hematopoietic cells and tissues and aberrantly expressed in cancer. Specifically, it is aberrantly-expressed in retrovirus-induced hematological tumors in mice, and is found to be rearranged in human Ewing's sarcoma and related primitive neuroectodermal tumors characterized by a t(11;22)(q24;q12) translocation. Our ongoing studies have shown that Fli1 is essential for embryonic development: Loss of Fli1 results in embryonic lethality due in part to absence of megakaryocytes and aberrant vasculogenesis. Loss of Fli1 also negatively impacts HSC differentiation, consistent with its role in the regulation of important stem cell regulatory genes. To date, limited attention has been directed towards elucidating the potential role of Fli1 in human hematological tumors cancers, including AML.
We therefore evaluated the level of Fli1 expression in AML using Reverse Phase Protein Array (RPPA) technology to measure Fli1 expression (along with 195 other antibodies) in marrow and blood samples from 511 newly diagnosed AML patients and 21 APL patients.
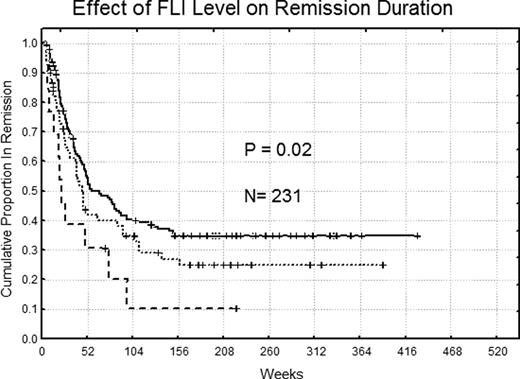
Compared to Fli1 protein levels in normal bone marrow CD34+ cells (n=11), expression was above normal in 32% of patients and below normal in 5%. Elevated Fli1 was not associated with any WHO classification, but was more common in FAB M1. Fli1 levels did not correlate with age, gender, performance status, or any single cytogenetic abnormality, but was negatively correlated with an antecedent hematologic disorder (p=0.002) and positively correlated with infection at the time of diagnosis (p=0.008). Levels were higher in patients with a NPM1 mutation (p=0.0005) or a FLT3-ITD abnormality (p< 0.00001). Expression was significantly lower at relapse compared to diagnosis in 49 paired samples (p= .02). Expression levels were similar in blood and marrow in 140 paired specimens (p=.65). Fli1levels were negatively correlated with 10 proteins (R >0.3) (integrinβ3, SRC, SRCp416, SRCp527, FAK, BCLXL AKTp308, FOXO3A, IGFBP2 and Fibronectin) and positively correlated (R >0.3) with 19 proteins (SMAD4, CREB, CREBp133, MTOR, ELKp383, TSC2, SSPB2, GAB2, PTEN and PTENp, HSP90, AKT, NPM1, NURR77, PRKR, TNK1, TAZp, BAX and DLX1). Fli1levels were not associated with remission attainment (p=.44), but remission duration (p=0.02) and overall survival (p=0.03) were significantly shorter for those with low (RemDur 22.6 and OS 45.2, weeks) or high Fli1(RemDur 40.3 and OS 35.4 weeks) compared to patients with normal levels of Fli1(RemDur 51.1 and OS 59.4 weeks). High Fli1 levels were prognostic in univariate analysis and was an independent prognostic factor in multivariate analysis along with age, unfavorable cytogenetics, favorable cytogenetics, gender, serum creatinine and FLT3mutation.
Both low and high expression of Fli1 protein are associated with poor outcome. While low levels of Fli1 may fail to properly regulate Fli1 target genes, elevated levels of Fli1 may not only affect expression of Fli1 target genes, but also aberrantly affect the expression of additional genes not normally responsive to Fli1. The correlation between high levels of FLi1 and high levels of AKT, mTOR, TSC2 and inactivated pPTEN suggest that targeting this signaling pathway might interfere with Fli1 expression.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal