Abstract
Abstract 1632
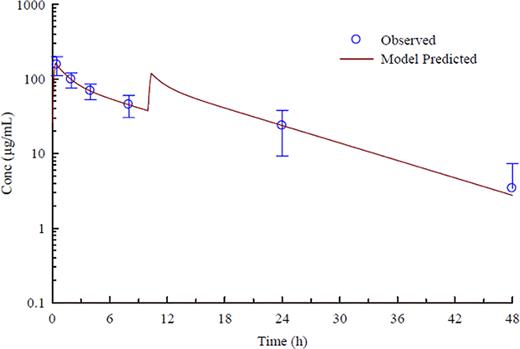
GMI-1070 is a pan-selectin inhibitor that targets E-, P-, and L-selectins and has shown activity in multiple animal models of disease. Sickle cell disease (SCD) is characterized by periodic vaso-occlusive (VOC) episodes in which cell adhesion and aggregation play a crucial role. GMI-1070 has previously been shown to restore blood flow and improve survival in a mouse model of VOC, and safety and PK have been evaluated in normal, healthy volunteers in phase 1. Here we report clinical, safety, and PK results from the first study of GMI-1070 in individuals with SCD. Methods: An open-label phase 1/2 study was performed, enrolling adults with SCD at steady state. GMI-1070 was administered in two IV doses given on the same day: 20 mg/kg in the first dose, followed 10 hours later by 10 mg/kg. Patients were evaluated for safety on days 0, 1, 2, 7 and 28, including adverse events (AEs), routine clinical labs, and clinical exam. Plasma and urine concentrations of GMI-1070 were measured on days 0, 1, and 2, and PK parameters calculated and compared with those from healthy volunteers. Results: Fifteen adults were enrolled at three centers; 13 with HbSS, 2 with HbSB0thal. All were African-American, 9 were male, mean age was 32 years (range 18–50), mean weight was 64.7 kg; 4 were on hydroxyurea. In the past year, 6 had experienced VOC requiring medical care; 2 had ACS; 2 required transfusions; and 1 had an episode of priapism. Five were hospitalized in the past year; 12 were hospitalized in the past 5 years. All subjects received both doses of study drug; all but one were followed for 28 days. The PK in adults with SCD was in good agreement with that in the controls. The elimination half-life of GMI-1070 averaged 7.73 ± 2.45 hours (Figure). Renal clearance averaged 18.0 ± 7.93 mL/min and accounted for essentially all elimination. Physical exam parameters after dosing were unchanged, and all infusions were well tolerated. Four subjects reported headache within 24 hours of dosing, all of which were mild or moderate and resolved within 24 hours. Two subjects experienced VOC not requiring hospitalization, at 2 and 4 weeks after dosing. One subject had worsening anemia requiring transfusion 5 days after dosing. Other adverse events typical of SCD were reported without apparent association with study drug; none were serious adverse events. Routine labs demonstrated no changes from baseline (Hb, reticulocytes, platelets, electrolytes, glucose, ALT, LDH, BUN, Cr, bilirubin, urinalysis) with the exception of white blood cell counts (WBC) and absolute neutrophil counts (ANC). At 24 hours, mean WBC change from baseline was 1.9K/mm3, or 20% (p=0.076, using parametric test with mixed model); mean ANC change was 2.7, or 67% (p=0.019); all returned to baseline by 7 days. One individual had marked leukocytosis 24 hours after dosing (from 10.4 to 28K/mm3), returning to baseline by day 7; no other effects were observed in this subject. Mean C-reactive protein (CRP) increased at 24 and 48 hours, returning to baseline by day 7. Two subjects had marked increases in CRP: one exhibited leukocytosis with dosing and the other had a high baseline WBC count. There was otherwise no apparent correlation between PK, WBC/ANC, hydroxyurea use, or adverse events. In conclusion, GMI-1070, a pan-selectin inhibitor, when administered to adults with SCD at steady state, has a similar safety and PK profile to that in healthy volunteers. However, SCD patients had moderate WBC and ANC increases at 24–48 hours after dosing, which return to baseline without other observed symptomatic adverse events. This study supports further evaluation of GMI-1070 for the treatment of vaso-occlusive crisis.
Observed (mean+/− SD) and model-predicted plasma concentrations of GMI-1070
Styles:GlycoMimetics: Consultancy, clinical trial sponsorship. Wun:GlycoMimetics: Consultancy, clinical trial sponsorship. De Castro:GlycoMimetics: clinical trial sponsorship. Telen:GlycoMimetics: Consultancy, clinical trial sponsorship. Kramer:GlycoMimetics: Consultancy. Flanner:GlycoMimetics: Employment, Equity Ownership. Magnani:GlycoMimetics: Employment, Equity Ownership. Thackray:GlycoMimetics: Employment, Equity Ownership. Off Label Use: This drug (GMI-1070) has not been approved for any clinical indication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal