Abstract
Abstract 153
The antiphospholipid (aPL) syndrome (APS) is an autoimmune, thrombotic condition reported to be responsible for approximately 10% of patients with thromboembolic disease. APS is characterized by autoantibodies against phospholipids or plasma proteins bound to phospholipids that inhibit phospholipid-dependent coagulation reactions in vitro. The diagnostic criteria for APS are defined by the presence of an aPL antibody and the occurrence of one or more thrombotic events, including arterial or venous thromboses, multiple fetal losses and thrombocytopenia. The current assays for diagnosing APS are empirically-derived and include the lupus anticoagulant assay and aPL immunoassays for anticardiolipin and anti-ß2 glycoprotein-I antibodies. These assays do not test report on disease mechanisms and have poor specificities, sensitivities and predictive values. To date, several different mechanisms have been proposed for APS, including aPL antibody-mediated disruption of a potent anticoagulant protein, annexin A5. Annexin A5 forms two-dimensional crystal shields over membranes that expose anionic phospholipids; the crystallization and shielding of phospholipid from availability for coagulation reactions is disrupted by aPL antibodies. Therefore, we investigated the prevalence of evidence for this mechanism in blood samples from aPL-positive and negative patients.
Clinical histories and laboratory data were obtained from adult patients with histories for venous thromboembolism (n=223), stroke (n=73) and pregnancy complications (n=178) that were evaluated for thrombophilia and from patients who were free of known disease (n=76). A novel mechanistic assay was performed for “annexin A5 resistance” (A5R), an assay that detects antibody-mediated interference with annexin A5 anticoagulant activity. Results were expressed as a percent prolongation of coagulation times by annexin A5. Differences between groups were compared using nonparametric Kruskal-Wallis test.
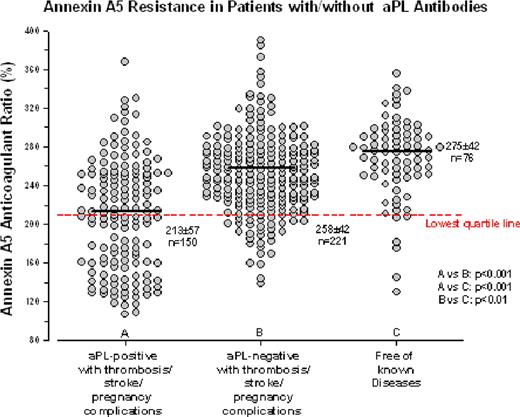
At a cut off A5R value at 210% - the lowest quartile value of A5R - 48% (72 out of 150) of patients in the aPL-positive thrombosis/stroke/pregnancy complication group had A5R values within the lowest quartile, in contrast to 15% (33 out of 221) of the aPL-negative thrombosis patients and 8% (6 out of 76) of the disease-free control. In aPL-positive patients, the A5R values (median±SD: 208±58%, n=196) were found to be significantly lower than in aPL-negative patients (247±46%, n=284, p<0.001). Furthermore, aPL-positive patients with any thrombotic event, including histories for venous thromboembolism, stroke and pregnancy complications, had significantly lower A5R values (213±57%, n=150) than aPL-negative patients with any thrombotic event (258±42%, n=221; p<0.001) and from patients who were free of known disease (275±42%, n=76; p<0.001).
Specifically, aPL-positive patients with a history of either venous thromboembolism (185±59, n=40) or pregnancy complications (233±73, n=25) had significantly lower A5R values than aPL-negative patients with either venous thromboembolism (250±62, n=62; p<0.01) or pregnancy complications (245±91, n=91; p<0.001).
This novel mechanistic assay identifies a significant subset of patients currently defined as aPL-positive as having evidence for aPL antibody-mediated resistance to annexin A5 anticoagulant activity. A5R may mark patients who have a propensity for venous thromboembolism and/or pregnancy complications. The identification of specific thrombogenic mechanisms in patients with APS through mechanistic assays opens the possibilities of improved diagnosis and targeted therapies for patients with this condition.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal