Abstract
Abstract 1459
Granulocyte-macrophage colony stimulating factor (GM-CSF) is a growth factor regulating proliferation and differentiation of hematopoietic stem and progenitor cells. Pegylation of murine GM-CSF (peg-mGM) has been shown to prolong its half-life. We have observed that patients transplanted with stem cell products mobilized by GM-CSF have a lower incidence of acute GvHD (Devine et al. BMT 2005), we hypothesized that peg-mGM mobilized stem cell products may be protective from acute GvHD in MHC mismatched murine models.
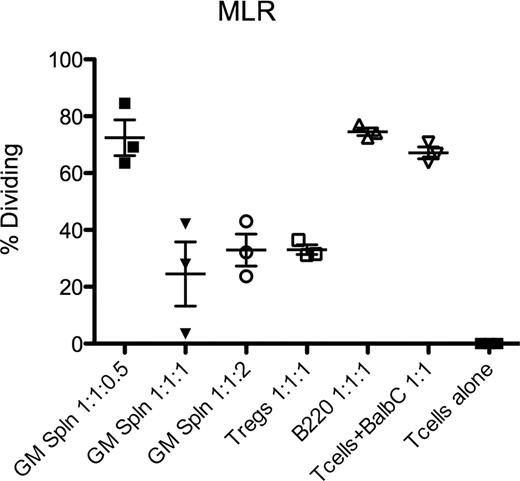
C57/Bl6 (B6) mice treated with peg-mGM (5mcg IP qd × 4) resulted in increased CFU-C by 26.5 fold compared to PBS treated mice (n=6, 95%CI 10.25–42.73). In addition, peg-mGM increased CD4+FoxP3+ Tregs in both blood and spleen by 2 fold compared to PBS (p<0.05). Importantly, the function of these peg-mGM Tregs, as measured in a mixed lymphocyte reaction (MLR) was found to be equivalent to Tregs from PBS treated control mice (t-test, n=3, p=0.27). Furthermore, we have observed an increased expression of Ki67 in murine Tregs in vivo in response to peg-mGM treatment consistent with peripheral expansion of Tregs. We hypothesized that a peg-mGM mobilized product would cause less graft versus host disease (GvHD). We have assessed the in vivo impact of peg-mGM on GvHD, by using the B6 to B6D2F1 acute GvHD model. B6 donors were treated with peg-mGM or PBS for 4 days; on the 5th day splenocytes were isolated. An equivalent dose of CD3+ T-cells along with congenic T cell depleted bone marrow was administered to recipients (n=5/group) that had undergone myeloablative conditioning (11 Gy TBI). We observed less weight loss and improved survival in the peg-mGM treated group (Log-rank chi-square 53.22, df=9, p<0.001). This effect may be explained by an increase in Tregs delivered in the peg-mGM group but also potentially to additional accessory cells that could be present in the graft and mediating an allo-suppressive response. We have phenotyped cells mobilized by peg-mGM and have observed a novel cellular phenotype consistent with a monocyte/macrophage mobilized in the peripheral blood that is SiglecH+, B220-, Ly6c+, CD11b+, GR-1 intermediate, and F4/80+/−. This subset appears within 2 days of the start of peg-mGM treatment and was not observed in mice mobilized with PBS, G-CSF or plerixafor. To evaluate the function of this novel population and its potential suppressor properties we performed an MLR. B6 mice were mobilized with peg-mGM for 4 days, on the 5th day whole splenocytes were isolated and single cell sorted for SiglecH+B220- population or B220+SiglecH- (control cells). These cells were incubated with irradiated Balb/C splenocytes (stimulators) and CFSE labeled congenic B6 CD4+CD25- T-conventional (Tcon) cells (responders) at varying ratios of stimulators:responders:suppresors. Freshly isolated naive Tregs were used as the positive control. At the end of 6 days of incubation wells containing the SiglecH+B220- cells at 1:1:1 or 1:1:2 ratios suppressed allo-reactive Tcon cells as potently as Tregs at a 1:1:1 ratio (t-test, n=3, p=0.49 and p=0.98 respectively) compared to no suppressor controls (t-test, n=3, p=0.02 and p=0.005 respectively)(Figure). Finally, we performed an ELISA for IFN-alpha on these sorted cells, which was not significantly increased (12 pg/mL) after stimulation with the TLR9 agonist, CpG.
These data suggest that reduced GvHD observed in mouse and man after infusion of GM-CSF mobilized stem cell products may be due to both in vivo expansion of Tregs and robust (<1% to 15–20%) mobilization of a novel population of myeloid suppressor cells. SiglecH, a member of the CD33 like immunoglobulin superfamily of lectin proteins, may identify a unique myeloid suppressor cell mobilized by GM-CSF in mice.
Schroeder:Genzyme: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal