Abstract
Abstract 1454
Recognizing p53 activation as the primary mechanism that underlies radiation-induced damage to bone marrow and GI track, we aim to explore the use of low dose arsenic-induced transient p53 inhibition as a novel strategy for radiotherapy protection.
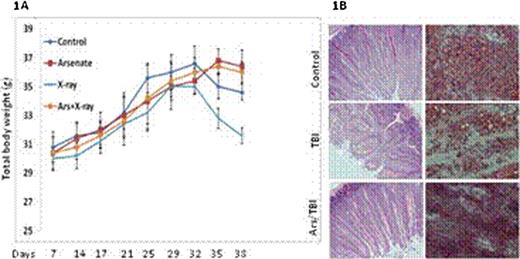
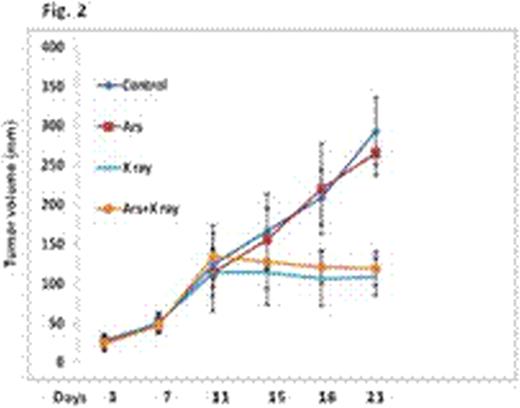
Expanding from our recent finding that low-dose of arsenic can suppress radiation-induced p53 activation and subsequent apoptosis, we used a mouse model to test the potential of arsenic in protection of bone marrow and GI track against TBI induced damage. Arsenic pretreatment was carried out by feeding sex-matched mice (4-6 weeks of age) with water with or without 1.0 mg/L sodium arsenic for three days. Mice were randomized into following groups; control; arsenic only; irradiation only; arsenic and irradiation. Mice were then irradiated with 2Gy TBI daily for 7 days. Radiation-induced bone marrow and GI track damages were analyzed histologically with H&E staining 4 weeks after last treatment. Animal body weight was monitored as a measure of toxicity. To test the prediction that arsenic would not provide protection for malignant cells because of their defect in p53, a lung carcinoma cell line NCI-H358 was used to generate a mouse xenograft model. Treatments were initiated three weeks after implantation when the lung carcinoma cells developed into readily visible tumor with an average volume of approximately 100 mm3. Tumor volumes were measured periodically. Tumor volume was calculated using the equation: (volume = length × width × depth × 0.5236 mm3). Two independent experiments were done and the tumor volumes are means ± SE from total of 10 mice per group.
Consistent with published results, mice treated with irradiation significantly lost their body weight. TBI-induced body weight loss was effectively prevented by arsenic pretreatment (Fig. 1A). In line with the data of body weight change, radiation treatments were associated with severe damages to small intestine and bone marrow cells, and remarkably, such damages were significantly reduced by low-dose arsenic pretreatment (Fig. 1B). The results together demonstrate the potential of low-dose arsenic to effectively protect bone marrow and GI track to radiation-induced damage.
In tumor xenograft models, the tumor volume of the control group continued to increase with time. Arsenic treatment did not have any detectable effect on growth of the implanted tumors. As expected, irradiation with 2Gy of TBI daily for 7 days resulted in marked tumor regression. Arsenic pretreatment showed little effect on irradiation-induced tumor suppression (Fig. 2), indicating that low-dose arsenic pretreatment does not detectably affect the efficacy of radiation, at least in the human lung carcinoma xenograft mouse model. Collectively, the results demonstrate that a brief treatment with low-dose arsenic is associated with a marked protection of bone marrow and GI track without compromising the ability of irradiation to kill carcinoma cells.
The animal studies have provided proof-of-principle for the use of low-dose arsenic in radiotherapy protection of bone marrow and GI track, serving as strong rationales to initiate clinical trials.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal