Abstract
Abstract 1423
Acquired factor VIII (FVIII) inhibitor is an uncommon condition, with an incidence of ≂f1.5 cases/million/year, as reported by Collins et al. (Blood 2007. 109: p. 1870–1877) in a two year observational study in the UK. However, this is a serious condition, characterized by auto-antibodies against circulating factor VIII and has high mortality from bleeding. Controlling the acute bleeding event with FVIII inhibitor bypassing agents like recombinant activated factor VII or activated prothrombin complex concentrates is of utmost importance. Equally important is the eradication of inhibitor, in order to restore normal hemostasis. There are several approaches for inhibitor eradication, including, but not limited to, the use of Corticosteroids, Cyclophosphamide, Azathioprine, Vincristine, IVIG, Cyclosporine, Mycophenolate and, recently, Rituximab, either singly, or, in different combinations. However, there is no single standard approach. We report 4 cases of acquired FVIII inhibitor from our center that were initially treated with factor VIII bypassing agents. Inhibitor eradication was achieved by using a combination of Rituximab, Prednisone and Cyclophosphamide.
Patient Demographics and Co-morbid conditions
| Patient . | Age (in years) . | Sex/Race . | Co-morbid conditions . |
|---|---|---|---|
| 1 | 44 | F/White | Multiple Sclerosis |
| 2 | 67 | M/White | Rheumatoid arthritis |
| 3 | 83 | F/White | Hypertension |
| 4 | 66 | M/Middle Eastern | Diabetes Mellitus, Obstructive uropathy |
| Patient . | Age (in years) . | Sex/Race . | Co-morbid conditions . |
|---|---|---|---|
| 1 | 44 | F/White | Multiple Sclerosis |
| 2 | 67 | M/White | Rheumatoid arthritis |
| 3 | 83 | F/White | Hypertension |
| 4 | 66 | M/Middle Eastern | Diabetes Mellitus, Obstructive uropathy |
Treatment Details
| Patient . | Hemoglobin(g/dl) Range:12.0-16.0 . | WBC(x109/l) . | PT(in seconds) Range:11.5-15.5 . | PTT(in seconds) Range:23.2-36 . | |
|---|---|---|---|---|---|
| 1 | 12.6 | 9.8 | 347 | 12.3 | 83 |
| 2 | 9.6 | 9.4 | 299 | 11.9 | 79 |
| 3 | 7.9 | 10.5 | 555 | 13.4 | 97.5 |
| 4 | 9.5 | 18.7 | 275 | 14.3 | 95.2 |
| Patient . | Hemoglobin(g/dl) Range:12.0-16.0 . | WBC(x109/l) . | PT(in seconds) Range:11.5-15.5 . | PTT(in seconds) Range:23.2-36 . | |
|---|---|---|---|---|---|
| 1 | 12.6 | 9.8 | 347 | 12.3 | 83 |
| 2 | 9.6 | 9.4 | 299 | 11.9 | 79 |
| 3 | 7.9 | 10.5 | 555 | 13.4 | 97.5 |
| 4 | 9.5 | 18.7 | 275 | 14.3 | 95.2 |
Treatment Details
| Patient . | Treatment Duration . | Cyclophosphamide . | Prednisone . | Rituximab . |
|---|---|---|---|---|
| 1 | 3 cycles of 28 days each | 500mg iv day 1 200mg PO days 2-5,q4weeks x 3 cycles | 100mg PO days 1-5,q 4 weeks x 3 cycles | 375mg/m2 day 1, then q 4 weeks x 3 doses |
| 2 | 6 cycles of 21 days each | 500mg iv day 1 200mg PO days 2-5,q 3 weeks x 6 cycles | 100mg PO days 1-5,q 3 weeks x6 cycles | 375mg/m2 day 1, then q 4 weeks x 3 doses |
| 3 | 4 cycles of 28 days each | 500mg iv day 1 200mg PO days 2-5,q 4 weeks x 4 cycles | 100mg PO days 1-5,q 4 weeks x 4 cycles | 375mg/m2 day 1, then q weekly x 3 doses; then q 4 week x 3 doses |
| 4 | 3 cycles of 28 days each | 500mg day 1 200mg PO days 2-5,q 4 weeks x 3 cycles | 100mg PO days 1-5,q 4 weeks x 4 cycles | 375mg/m2 day 1, then q weekly x 3 doses; then q 4 week x 3 doses |
| Patient . | Treatment Duration . | Cyclophosphamide . | Prednisone . | Rituximab . |
|---|---|---|---|---|
| 1 | 3 cycles of 28 days each | 500mg iv day 1 200mg PO days 2-5,q4weeks x 3 cycles | 100mg PO days 1-5,q 4 weeks x 3 cycles | 375mg/m2 day 1, then q 4 weeks x 3 doses |
| 2 | 6 cycles of 21 days each | 500mg iv day 1 200mg PO days 2-5,q 3 weeks x 6 cycles | 100mg PO days 1-5,q 3 weeks x6 cycles | 375mg/m2 day 1, then q 4 weeks x 3 doses |
| 3 | 4 cycles of 28 days each | 500mg iv day 1 200mg PO days 2-5,q 4 weeks x 4 cycles | 100mg PO days 1-5,q 4 weeks x 4 cycles | 375mg/m2 day 1, then q weekly x 3 doses; then q 4 week x 3 doses |
| 4 | 3 cycles of 28 days each | 500mg day 1 200mg PO days 2-5,q 4 weeks x 3 cycles | 100mg PO days 1-5,q 4 weeks x 4 cycles | 375mg/m2 day 1, then q weekly x 3 doses; then q 4 week x 3 doses |
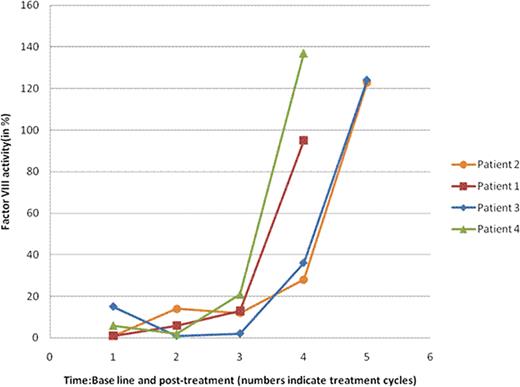
Factor VIII Activity (in%): Baseline and Post-Treatment (Range: 50-180%)
| Patient . | Baseline . | Postcycle1 . | Postcycle2 . | Postcycle3 . | Postcycle4 . | Postcycle5 . | Postcycle6 . |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 6 | 13 | 95 | |||
| 2 | 1 | NA | NA | 14 | 12 | 28 | 123 |
| 3 | 15 | 1 | 2 | 36 | 124 | ||
| 4 | 6 | 2 | 21 | 137 |
| Patient . | Baseline . | Postcycle1 . | Postcycle2 . | Postcycle3 . | Postcycle4 . | Postcycle5 . | Postcycle6 . |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 6 | 13 | 95 | |||
| 2 | 1 | NA | NA | 14 | 12 | 28 | 123 |
| 3 | 15 | 1 | 2 | 36 | 124 | ||
| 4 | 6 | 2 | 21 | 137 |
NA: not available
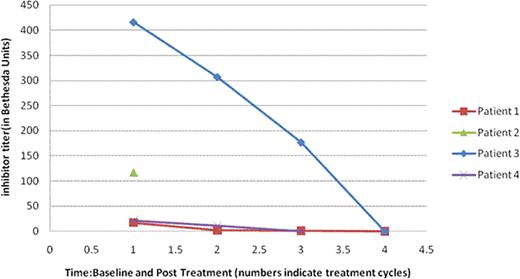
Factor VIII inhibitor titer (in Bethesda Units): Baseline and Post-Treatment (Normal: Undetectable)
| Patient . | Baseline . | Postcycle1 . | Postcycle2 . | Postcycle3 . | Postcycle4 . | Postcycle5 . | Postcycle6 . |
|---|---|---|---|---|---|---|---|
| #1 | 17.2 | 1.7 | 1.2 | Undetectable | |||
| #2 | 117 | NA | NA | NA | NA | NA | NA |
| #3 | 416 | 307 | 177 | Undetectable | |||
| #4 | 20.9 | 10.8 | Undetectable |
| Patient . | Baseline . | Postcycle1 . | Postcycle2 . | Postcycle3 . | Postcycle4 . | Postcycle5 . | Postcycle6 . |
|---|---|---|---|---|---|---|---|
| #1 | 17.2 | 1.7 | 1.2 | Undetectable | |||
| #2 | 117 | NA | NA | NA | NA | NA | NA |
| #3 | 416 | 307 | 177 | Undetectable | |||
| #4 | 20.9 | 10.8 | Undetectable |
NA: not available
Table 6: Follow up
| Patient . | Time from last treatment (in months) . | Most recent factor VIII activity (Range: 50-180%) . | Inhibitor titer . |
|---|---|---|---|
| #1 | 9 | 100% | NA |
| #2 | 6 | 126% | NA |
| #3 | 16 | 105% | Undetectable |
| #4 | 3 | 55% | Undetectable |
| Patient . | Time from last treatment (in months) . | Most recent factor VIII activity (Range: 50-180%) . | Inhibitor titer . |
|---|---|---|---|
| #1 | 9 | 100% | NA |
| #2 | 6 | 126% | NA |
| #3 | 16 | 105% | Undetectable |
| #4 | 3 | 55% | Undetectable |
NA: not available
Complete and sustained remission was achieved in all 4 patients (100%).All 4 patients tolerated the therapy well. None of the patients had significant adverse effects like neutropenia, hemorrhagic cystitis or other complications, either immediately or during follow up. Thus, the combined use of Cyclophosphamide, Rituximab and Prednisone seems to be an effective and safe regimen for inhibitor eradication in patients with acquired FVIII inhibitor.
Off Label Use: Rituximab.For th purpose of auto-antibody supression.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal