Abstract
Abstract 140
Peripheral T-cell lymphomas (PTCLs) have poor outcomes, and there is a lack of prognostic biomarkers and therapeutic targets to guide treatment. We recently proposed that the transcription factor, interferon regulatory factor-4 (IRF4, also called multiple myeloma oncogene-1 [MUM1]), might be oncogenic in PTCLs based on its expression in association with translocations between IRF4 and the T-cell receptor gene, TRA@. IRF4 is a therapeutic target in multiple myeloma, where high expression is a poor prognostic factor. In addition, at least two germline IRF4 single nucleotide polymorphisms (SNPs), rs12203592 and rs872071, are associated with IRF4 expression and disease risk and progression in various lymphoid neoplasms. However, the prognostic effects of IRF4/MUM1 expression and IRF4 SNPs in PTCLs are unknown.
Forty seven newly diagnosed PTCL patients with available tissue were identified from the University of Iowa/Mayo Clinic Lymphoma SPORE Molecular Epidemiology Resource. There were 5 anaplastic large cell lymphomas, 14 cutaneous T-cell lymphomas (CTCLs), 6 cytotoxic T-cell lymphomas (cytTCLs: 4 extranodal NK/T-cell and 2 enteropathy type), and 22 PTCLs, not otherwise specified (NOS). Patients were diagnosed between September 2002 and February 2008 and systematically followed through March 2010 for overall survival (OS). Tumor cell IRF4/MUM1 expression was examined by immunohistochemistry on paraffin tissue sections (MUM1p clone; Dako). Positivity was defined as >30% of tumor cells with nuclear staining, as in previous studies. rs12203592 and rs872071 were genotyped in peripheral blood DNA. Cox regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for both IRF4/MUM1 expression and SNPs with OS. Chi-squared tests were used to assess the relationship between IRF4/MUM1 expression and IRF4 SNPs.
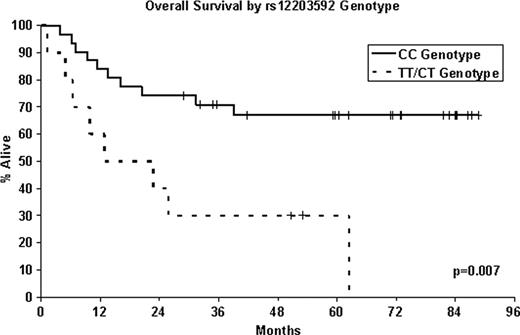
The median age at diagnosis was 60 years (range, 24–88). At a median follow-up of 62 months (range, 29–89), 22 (47%) of the patients had died. Fifteen (32%) of the 47 T-cell patients had IRF4/MUM1 positive tumors. IRF4/MUM1 positivity was associated with poorer overall survival (HR=4.3; 95% CI: 1.8–10.2; p=0.0008). This association was seen across PTCL subtypes, including PTCL, NOS (HR= 6.5; 95% CI: 1.5–27.7; p=0.01), CTCL (HR=13.4; 95% CI: 1.2–150.1; p=0.03), and cytTCL (HR=5.8; CI: 0.5–65.9; p=0.15). The minor allele (T) in SNP rs12203592 was positively associated with IRF4/MUM1 positivity in a dominant model, with IRF4/MUM1 expression in 60% of patients with CT or TT genotypes compared to 19% of patients with CC genotype (p=0.01). Patients with the CT/TT genotype at rs12203592 also had inferior overall survival (HR=3.7; 95% CI: 1.4–9.5; p=0.007). The rs872071 SNP showed no significant association with either IRF4/MUM1 expression (p=0.38) or overall survival (p=0.71).
This study is the first to demonstrate that IRF4/MUM1 expression is poor prognostic factor in PTCLs. This association was observed across PTCL subtypes, including the most common subtype, PTCL, NOS. IRF4/MUM1 expression and poor survival in our patients also were associated with the minor allele (T) in the IRF4 SNP rs12203592; these findings are consistent with previous in vitro data showing the major allele (C) represses IRF4 promoter activity. Interestingly, rs872071 was not associated with IRF4/MUM1 expression or prognosis, similar to findings in multiple myeloma. In contrast, rs872071 is a risk and prognostic factor in chronic lymphocytic leukemia and classical Hodgkin lymphoma, diseases in which IRF4/MUM1 expression has been associated with favorable prognosis. IRF4/MUM1 expression is a poor prognostic factor in PTCLs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal