Abstract
Abstract 1386
Hyperacute TLS, defined as a rise in uric acid, potassium, lactate dehydrogenase (LDH), and/or phosphate that occurs within 4.5 hours and peaks 24–48 hours after flavopiridol, has been described in up to 40% of pts with CLL treated with this agent.
A retrospective analysis of 116 pts with relapsed or refractory CLL treated with single agent flavopiridol at the Ohio State University on phase I or II protocols was conducted to determine predictive factors for TLS. Pts received flavopiridol 30–50 mg/m2 bolus + 30–50 mg/m2 4-hour continuous IV infusion (CIVI) on days 1, 8, 15, and 22 every 35 days or days 1, 8, and 15 every 28 days.
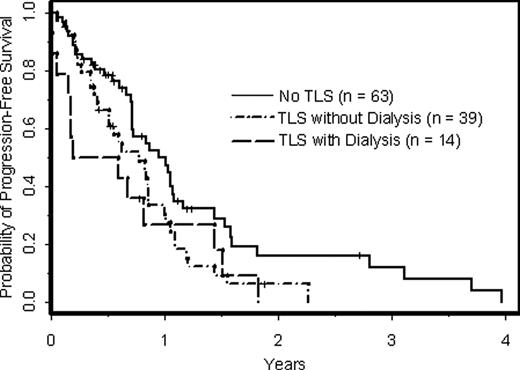
In 116 pts, the median age was 60 (range, 31–84), median number of prior therapies was 4 (range, 1–14), 69% pts were male, 79% were Rai stage III-IV, 53% had bulky disease ≥ 10 cm, 52% had splenomegaly, and 69% had del(17p) and/or del(11q). Median pre-treatment laboratory values included B2-microglobulin (B2M) 4.4 (range, 0.8–14.9), absolute lymphocyte count (ALC) 7134/mm3 (range, 0–266,310), white blood cell count (WBC) 13,950/mm3 (range, 1,300-314,500), and LDH 199 U/L (range, 102–654). The incidence of TLS was 46% (95% CI: 36%-55%), with 14 of 53 pts (26%) with TLS requiring dialysis. In univariable analyses using logistic regression, variables associated with the occurrence of TLS were female gender (p<0.001), number of prior therapies (p<0.001), Rai stage III-IV (p<0.001), bulky disease ≥ 10 cm (p<0.001), splenomegaly (p=0.04), del(11q) (p=0.03), ALC (p=0.004), WBC (p<0.001), B2M (p<0.001), and LDH (p=0.003). 72% of females, 36% of males, 59% pts with lymphadenopathy ≥ 10 cm, 31% pts with lymphadenopathy < 10 cm, 55% pts with del(11q), and 35% pts with del(17p) developed TLS. Median B2M and WBC values in pts without TLS were 3.1 and 8,300/mm3 compared to 5.3 and 27,000/mm3 in pts with TLS, respectively. In a multivariable analysis using limited backwards selection (Table 1), female gender, bulky adenopathy ≥ 10 cm, WBC, and B2M were significantly associated with TLS (p<0.05). Notably, only 3 of 24 pts with Rai stage I/II disease developed TLS, and the small numbers of Rai stage I-II pts precluded the use of this variable in the multivariable analysis. Therefore, the multivariable analysis was restricted to pts with Rai stage III/IV (n=92), and the same 4 variables in Table 1 remained significantly associated with TLS. TLS occurred in all pts (n=8) with WBC > 150,000/mm3, 75% pts with WBC 100–150,000, and 38% pts with WBC < 100,000/mm3. TLS rates were 74% and 19% in pts with B2M above and below the median (4.4), respectively. In a secondary analysis, we examined if peak flavopiridol and its glucoronide metabolite (flavo-G) levels correlated with TLS or gender. In a subset of 85 pts with available data, flavo-G levels were associated with TLS (p=0.001), but this was independent of pt gender. When peak flavo-G levels were distributed into quartiles, 50% women in the lowest quartile developed TLS compared to 93% in the highest quartile. Likewise, 14% of men with the lowest flavo-G levels developed TLS as opposed to 57% in the highest quartile. With respect to pt outcomes, 49% with TLS and 44% without TLS responded to flavopiridol. In a multivariable model controlling for number of prior treatments, cytogenetic risk group, Rai stage, age, and gender, response rates were not significantly different (p=0.34) in patients with and without TLS. However, overall survival (OS) and progression-free survival (PFS) were inferior in pts with TLS (p=0.03 and p=0.04, respectively). Eighty-six pts have died, including 13 of 14 pts with TLS who required dialysis. For pts with TLS that did not require dialysis, OS was not significantly different (p=0.88), although PFS was still worse in this subgroup (p=0.03, Figure 1).
Female pts and pts with B2M ≥ 4.4, WBC ≥ 100,000/mm3, or bulky adenopathy ≥ 10 cm were at highest risk and should be monitored for hyperacute TLS with flavopiridol. TLS does not appear to be predictive of response or improved PFS in pts receiving flavopiridol.
Supported by NCI K23 CA109004, NCI U01 CA076576, NCI N01 CM62207, LLS SCOR 7080-06, and the D. Warren Brown Foundation.
| Risk Factor . | Odds Ratio . | 95% CI . | P value . |
|---|---|---|---|
| Female vs. Male | 9.5 | 3.0–30.3 | <0.001 |
| Bulky lymphadenopathy ≥ 10 cm | 3.2 | 1.1–9.6 | 0.04 |
| WBC, 50 unit increase | 1.9 | 1.0–3.2 | 0.02 |
| B2M, 1 unit increase | 1.5 | 1.2–1.9 | <0.001 |
| Risk Factor . | Odds Ratio . | 95% CI . | P value . |
|---|---|---|---|
| Female vs. Male | 9.5 | 3.0–30.3 | <0.001 |
| Bulky lymphadenopathy ≥ 10 cm | 3.2 | 1.1–9.6 | 0.04 |
| WBC, 50 unit increase | 1.9 | 1.0–3.2 | 0.02 |
| B2M, 1 unit increase | 1.5 | 1.2–1.9 | <0.001 |
PFS of CLL pts with respect to occurrence of TLS after treatment with flavopiridol.
PFS of CLL pts with respect to occurrence of TLS after treatment with flavopiridol.
Off Label Use: The efficacy of the cyclin dependent kinase inhibitor, flavopiridol is under investigation in CLL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal