Abstract
Abstract 1364
In light chain (AL) amyloidosis, as well as in multiple myeloma, response to treatment is increasingly being used as a surrogate endpoint in clinical trials. In 2005 a consensus statement of the International Society of Amyloidosis (ISA) established the criteria for hematologic and organ response. Since then, several studies emphasized the prognostic relevance of the measurement of the amyloidogenic precursor, the circulating free light chain (FLC). Moreover, it was reported that patients who with treatment achieved decreases in the cardiac biomarker N terminal natriuretic peptide type B (NT-proBNP) had longer survival, although echocardiographic criteria of response were not attained.
The ISA Consensus Panel reconvened in 2010 to update hematologic and organ response criteria. The panel felt that any new criteria should be validated in a large patient population. Thus, we systematically gathered from 7 referral centers in Europe and in the United States a cohort of 649 patients with systemic AL amyloidosis who had been evaluated for hematologic and organ responses at diagnosis and 6 months after treatment initiation, excluding patients who died earlier. At diagnosis, 430 patients (66%) had heart involvement, 377 (58%) had NT-proBNP ≥650 ng/L, 455 (70%) had renal involvement (95, 15%, with glomerular filtration rate <30 mL/min) and 100 (15%) had liver involvement. Two-hundred eighty-nine patients (44%) were treated with melphalan and dexamethasone, 118 (18%) received thalidomide based therapy, 73 (11%) underwent autologous stem cell transplant, 35 (5%) were treated with regimens including lenalidomide, 20 (3%) received bortezomib-based therapy, and the rest received other alkylating agents, nucleoside analogues or dexamethasone. The median follow-up of living patients was 24 months, and 233 patients (34%) died. The ability of response criteria to identify patients who died was compared by evaluating the areas under Receiver Operator Characteristic curves based on death at 1 year, and by calculating the Harrell C statistic and the Royston explained variation. Survival was calculated from the time of evaluation of response.
We maintained the category of complete response (CR: negative serum and urine immunofixation, normal FLC kappa/lambda ratio and normal marrow studies) and examined candidate criteria for partial (PR) and very good partial responses (VGPR), based on percentage changes or absolute values achieved after treatment of involved (amyloidogenic) FLC (iFLC), and alternatively on the difference between iFLC and uninvolved FLC (dFLC). With respect to cardiac response and progression, NT-proBNP-based criteria were defined as a decrease or an increase of both >30% and >300 ng/L, and a threshold of evaluability based on NT-proBNP baseline level >650 ng/L was chosen.
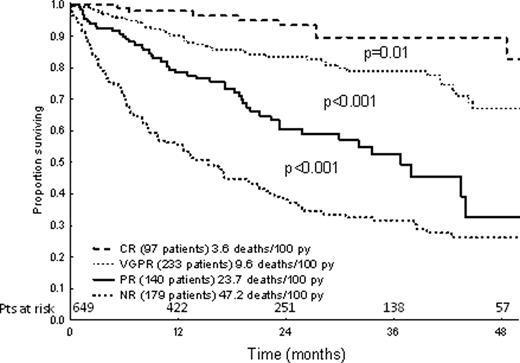
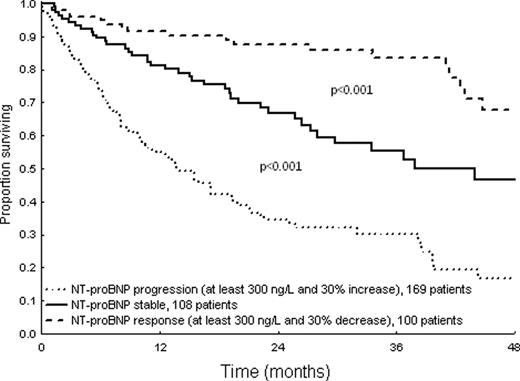
The most powerful criteria for PR were those based on dFLC percent decrease, and a 50% cutoff was preferred because of easier clinical use. Among candidate criteria for VGPR, the best were based on iFLC absolute value achieved after therapy, but the performance of those based on dFLC absolute value was only slightly lower. Therefore, a definition of VGPR based on dFLC (<40 mg/L) was adopted for the sake of harmonization with the dFLC-based definition of PR. The adopted hematologic response criteria and their prognostic significance are reported in Table 1. These criteria identified 4 groups with significantly different survivals (Figure 1). Also the proposed criteria of NT-proBNP response and progression were significantly associated with survival (Figure 2).
Our 2010 revised consensus criteria for hematologic response include maintenance of the definition of CR, and with use of dFLC re-casting the definition of PR and introducing a VGPR category, and for cardiac response and progression introducing the use of changes in NT-proBNP levels. In a further analysis we will address the definition of measurable dFLC at baseline and evaluate the applicability of the response criteria to earlier evaluation of response. The revised criteria improve the framework for clinical research in AL.
| Criteria . | HR (95%CI) . | P . |
|---|---|---|
| CR (negative serum and urine and normal FLC ratio) | 1 | – |
| VGPR (dFLC <40 mg/L) | 2.67 (1.26–5.66) | 0.01 |
| PR (dFLC decrease >50%) | 6.24 (2.96–16.15) | <0.001 |
| NR | 12.34 (6.03–25.35) | <0.001 |
| Criteria . | HR (95%CI) . | P . |
|---|---|---|
| CR (negative serum and urine and normal FLC ratio) | 1 | – |
| VGPR (dFLC <40 mg/L) | 2.67 (1.26–5.66) | 0.01 |
| PR (dFLC decrease >50%) | 6.24 (2.96–16.15) | <0.001 |
| NR | 12.34 (6.03–25.35) | <0.001 |
For the model p<0.001, Harrel C = 0.72, Royston explained variation = 0.33
Off Label Use: Thalidomide, lenalidomide, bortezomib for systemic AL amyloidosis. Dispenzieri:The Binding Site: Honoraria. Gertz:Celgene: Honoraria; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dimopoulos:Ortho-Biotech: Honoraria; Celgene: Honoraria; Millennium: Honoraria. Merlini:Millennium: Honoraria; Ortho-Biotech: Honoraria.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal