Abstract
Abstract 1292
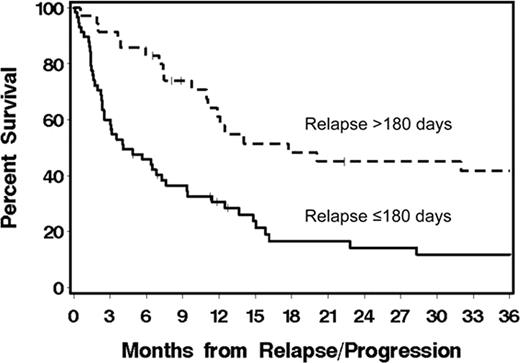
Allogeneic HCT after nonmyeloablative conditioning can cure a substantial number of patients (pts) with CLL or NHL. However, progression/relapse remains a problem. Here, we describe outcomes among 93 (of a total 337) patients with CLL/NHL who progressed or relapsed after allogeneic HCT on multi-institutional clinical trials using nonmyeloablative conditioning with 2 Gy total body irradiation alone (19%) or with 90 mg/2 fludarabine (81%). Grafts, carried out between December 1997 and June 2009, were either from HLA-matched related (n=55), unrelated (n=30) or HLA-mismatched donors (n=8). Median age at HCT was 56 (range 18–68) years. Pts had diagnoses of CLL (39%), mantle cell lymphoma (MCL, 13%), indolent (11%), or aggressive NHL (37%). At HCT, 31%, 18%, 35%, and 16% of pts had HCT-comorbidity index (CI) scores of 0, 1, 2–3, and ≥4, respectively. After HCT, 53% and 35% of pts experienced grades II-IV acute and extensive chronic graft-versus-host disease (GVHD), respectively. Median time to progression/relapse was 100 (range 6–2846) days. Median follow up for 21 surviving patients was 48 (range 3–103) months from time of HCT and 30 (range 1.6–92) months from time of progression/relapse. Overall survival (OS) at 1-year from time of progression/relapse was 40% for all pts. OS was 31% vs. 61% (p = 0.0009) for pts who relapsed at ≤180 vs. >180 days (Figure). Early vs. late relapsing pts had more frequent HCT-CI scores of >1 (59% vs. 37%) and aggressive NHL histology (48% vs. 17). Pts received either 0 (13%), 1 (22%), 2 (39%), or 3–5 (26%) different post-relapse treatment interventions. Interventions included (Table 1) rapid taper of immunosuppressive medications (28%), monoclonal antibody (56%), chemotherapy (51%), radiotherapy (22%), donor lymphocyte infusion (24%), and/or 2nd allogeneic HCT (7%). OS at 1-year was greater among pts who received monoclonal antibody (mainly rituximab) as their initial intervention modality (Table 2). In Cox regression models, relapse within ≤180 days from HCT (HR: 3.3, p=0.001), unrelated grafts (HR: 1.77, p=0.05), and pt age >55 years (HR: 2.08, p=0.007) independently predicted increased overall mortality, while initial treatment with either monoclonal antibody (HR: 0.25, p=0.0003), DLI (HR: 0.25, p=0.002), chemotherapy (HR: 0.36, p=0.01), or combined chemotherapy and monoclonal antibody (HR: 0.29, p=0.01) were associated with lessened mortality. Pre-transplant HCT-CI scores, the presence of acute or chronic GVHD, and histology did not impact mortality. Results show that pts who relapsed >180 days after HCT had relatively longer survival than pts who relapsed earlier. The initial use of rituximab-based salvage therapy was associated with the best survival. Pts who relapsed within the first 180 days after HCT had a relatively poor outcome. Rapid taper of immunosuppressive medications was of unclear benefit. Investigating novel immunomodulatory agents ± rituximab for pts with early relapse after HCT is warranted.
Overall survival among pts with CLL/lymphoma who relapsed/progressed ≤180 vs. >180 (p=0.0009) days after nonmyeloablative allogeneic HCT.
Overall survival among pts with CLL/lymphoma who relapsed/progressed ≤180 vs. >180 (p=0.0009) days after nonmyeloablative allogeneic HCT.
Interventions after relapse of CLL/NHL following nonmyeloablative HCT
| Interventions . | HCT-to-relapse interval . | ||
|---|---|---|---|
| ≤180 days (n=58), % . | >180 days (n=35), % . | ||
| # | 0 | 16 | 9 |
| 1 | 21 | 23 | |
| 2 | 38 | 43 | |
| 3-5 | 25 | 23 | |
| Interventions . | HCT-to-relapse interval . | ||
|---|---|---|---|
| ≤180 days (n=58), % . | >180 days (n=35), % . | ||
| # | 0 | 16 | 9 |
| 1 | 21 | 23 | |
| 2 | 38 | 43 | |
| 3-5 | 25 | 23 | |
| Included . | Rapid taper of immunosuppressive medications . | 43 . | 3 . |
|---|---|---|---|
| Monoclonal antibody | 47 | 71 | |
| Chemotherapy | 49 | 60 | |
| Radiotherapy | 21 | 23 | |
| DLI | 19 | 32 | |
| 2nd HCT | 7 | 6 |
| Included . | Rapid taper of immunosuppressive medications . | 43 . | 3 . |
|---|---|---|---|
| Monoclonal antibody | 47 | 71 | |
| Chemotherapy | 49 | 60 | |
| Radiotherapy | 21 | 23 | |
| DLI | 19 | 32 | |
| 2nd HCT | 7 | 6 |
Overall survival 1-year post-relapse as stratified by the first modality of treatment.
| Initial intervention modality . | Overall survival at 1-year, % . | p-value . | |
|---|---|---|---|
| Rapid taper of immunosuppressive medications | Yes | 39 | 0.24 |
| No | 44 | ||
| Monoclonal antibody | Yes | 57 | 0.02 |
| No | 34 | ||
| Chemotherapy | Yes | 40 | 0.99 |
| No | 43 | ||
| DLI | Yes | 55 | 0.50 |
| No | 40 | ||
| Chemo-combined immunotherapy | Yes | 47 | 0.99 |
| No | 42 | ||
| Initial intervention modality . | Overall survival at 1-year, % . | p-value . | |
|---|---|---|---|
| Rapid taper of immunosuppressive medications | Yes | 39 | 0.24 |
| No | 44 | ||
| Monoclonal antibody | Yes | 57 | 0.02 |
| No | 34 | ||
| Chemotherapy | Yes | 40 | 0.99 |
| No | 43 | ||
| DLI | Yes | 55 | 0.50 |
| No | 40 | ||
| Chemo-combined immunotherapy | Yes | 47 | 0.99 |
| No | 42 | ||
Off Label Use: Fludarabine and total body irradiation are off-label drug use for hematopoietic cell transplantation conditioning regimens.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal