Abstract
Abstract 1248
Iron overload is an important adverse prognostic factor in patients undergoing allogeneic hematopoietic stem cell transplantation (allo-SCT). Many studies have shown that elevated pre-transplant serum ferritin levels are associated with an increased risk of morbidity and mortality after allo-SCT. Conversely, a couple of other studies have suggested that elevated pre-transplant ferritin levels are associated with a low incidence of the chronic graft-versus-host disease (GVHD). To verify these apparently paradoxical findings, we analyzed the association between pre-transplant serum ferritin levels and clinical outcomes of allo-SCT.
We retrospectively studied 161 consecutive patients (age, 16–65 years; median age, 46 years) who underwent their first allo-SCT for hematologic diseases at the Kyoto University Hospital between September 2004 and April 2010. The primary diseases were myeloid (n = 87) and lymphoid malignancies (n = 68), and non-malignant diseases (n = 6). Patients with acute leukemia in complete remission, untreated MDS, CML in the chronic phase, or non-malignant diseases were considered to have standard-risk diseases (n = 96), whereas those with any other hematologic disease status were considered to have high-risk diseases (n = 65). Stem cells were obtained from the bone marrow (BM) or peripheral blood (PB) of HLA-matched related donors (n = 56) and partially matched related donors (n = 18), BM of unrelated donors (n = 65), and cord blood of unrelated donors (n = 22). Conventional myeloablative regimens were used in 99 patients while reduced-intensity regimens were used in 62. The primary endpoint was the cumulative incidence of chronic GVHD, which is defined according to the Seattle criteria, while the secondary endpoints were overall survival, acute GVHD, treatment-related mortality (TRM), and relapse rate. For statistic analyses, cumulative incidence curves were used in a competing-risks setting to determine the incidence of GVHD, TRM, and relapse rate, whereas the Kaplan–Meier method was used to determine survival rate. The Fine and Gray proportional-hazards model for the sub-distribution of a competing risk and the Cox proportional-hazards regression model were used as appropriate. Factors evaluated in the analysis included the recipient's age, sex, diagnosis, disease status at the time of transplant, source of stem cells, conditioning regimen, GVHD prophylaxis, serum ferritin levels (<900 vs. ≥900 ng/mL), and serum CRP levels (<0.2 vs. ≥0.2 mg/dL). Only ferritin levels, CRP levels (which is associated with ferritin levels), and those factors with P-values of <0.10 in the univariate analysis were included in the multivariate analysis.
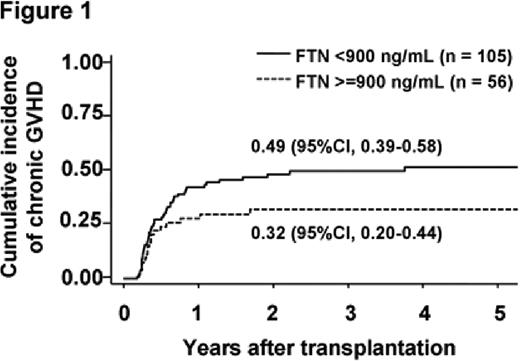
There was no significant difference in patient characteristics between the low-ferritin group (n = 105) and the high-ferritin group (n = 56). The median follow-up period among survivors was 38 months (range, 2–113 months). The cumulative incidence of chronic GVHD was lower in the high-ferritin group (32%) than in the low-ferritin group (49%) (P = 0.080) (Figure 1). In the multivariate analysis, serum ferritin level was significantly associated with the incidence of chronic GVHD (hazard ratio, 0.59; P = 0.045). Even when the serum ferritin level was treated as a continuous variable, this association remained significant. A trend toward a higher incidence of grade 3–4 acute GVHD was observed in the high-ferritin group; however, this association was not statistically significant. TRM was significantly higher in the high-ferritin group, but the relapse rate was not different between the 2 groups. High serum ferritin levels, the male sex, and high-risk diseases were significantly associated with a lower overall survival in the multivariate analysis.
In our cohort, elevated pre-transplant ferritin levels were significantly associated with a lower incidence of chronic GVHD. Conversely, patients in the high-ferritin group tended to have a high incidence of severe acute GVHD. These patients had lower overall survival probably due to a higher incidence of TRM. Further clinical and biological studies on the immunomodulatory effects of iron or iron-related molecules, such as ferritin, may provide clues on the prophylaxis and management of acute and chronic GVHD. For this purpose, studies on post-transplant iron status in association with acute and chronic GVHD should also be conducted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal