Abstract
Abstract 123
Lenalidomide is an effective agent in the treatment of MDS particularily in those possesing the deletion 5q (del5q) abnormality. The disease phenotype is partly related to loss of genetic material found in the commonly deleted region (CDR) on the long arm of chromosome 5. Recently implicated genes include miRNA-143, 145 and 146 all of which appear to play a role in innate immune signalling. In this study we examined whether upregulation of miRNAs in pretreatment CD34+ marrow cells from patients with MDS (both del5q and non-del5q) exposed to lenalidomide in vitro predicts for clinical response to the drug.
In total 31 pre-treatment patient samples were obtained from three North American centres. 11 patients' samples were positive for del5q. All patients had received lenalidomide as per local protocols. Clinical response was defined as per the International Working Group 2000 criteria. Clinical data were available for 26 patients. Hematologic improvement (HI), specifically the eythroid response (HI-E), was examined as the main clinical endpoint. Changes in miR-143, miR-145, miR-146a and miR-146b expression in CD34+ cells after in vitro lenalidomide exposure (10uM for 48 hours) were examined. The CD34- fraction was also analyzed. dCT values were normalized to 5S. A ≥ 1.5 fold change in miRNA expression was considered significant.
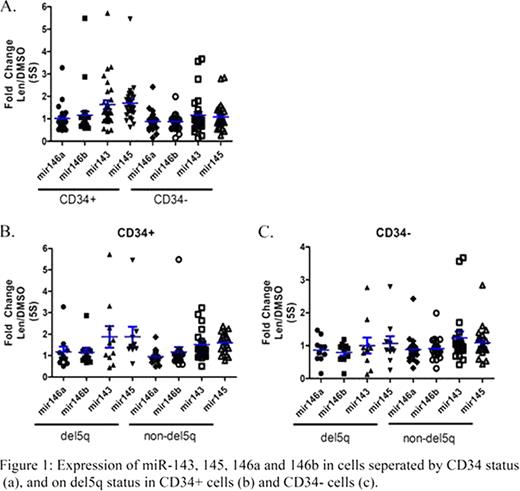
For the entire cohort mean fold changes in miRNA-143 and 145 post lenalidomide exposure were 1.6 and 1.7, respectively. Little change was seen in miR-146a and miR-146b expression (mean fold change 1.1 and 1.2, respectively). No significant increase was seen in any of the examined miRNA in the CD34 - fraction (Figure 1a). When separated based on del5q status both del5q and non-del5q groups showed a > 1.5 fold increase in expression of miR-143 and miR-145. No significant change in either miR-146a or miR-146b expression was seen (Figure 1b). In the CD34- fraction no significant change was seen in any of the examined miRNAs, irrespective of del5q status (Figure 1c). Examining HI-E, major responses (MR) were seen in 42% of patients (87.5% in the the del 5q cohort (7/8 had a MR) and 22.2% for the non-del5q cohort (4/18 had a MR)). Correlation with clinical response and miRNA expression in the CD34+ fraction is shown in Table I. In the del5q cohort MR was associated with an increase in both miR-143 and miR-145. No correlation with miRNA expression and clinical response was seen in the non del5q cohort.
Exposure of CD34+ cells from patients with MDS to lenalidomide results in up regulation of miR-143 and miR-145, irrespective of del5q status. Clinically, upregulation of miR-143 and miR-145 correlates with a HI-E in patients with del5q and may be predictive of response to therapy. Interestingly, the non-responder in the del5q group failed to upregulate miR-145. A similar gene induction pattern was seen in the non-del5q cohort, yet no correlation was seen with clinical outcome. Given that non-del5q patients do not harbor any abnormalities within the CDR of chromosome 5 these miRNAs are unlikely to play a role in their disease phenotype, nor in their response to lenalidomide.
Correlation between clinical response and miRNA expression.
| Hematologic Improvement - Erythroid (n) . | miR143 increased (n) . | miR145 increased (n) . |
|---|---|---|
| Del5q | ||
| MR (7) | 4 | 5 |
| No MR (1) | 1 | 0 |
| Total (8) | 5 | 5 |
| Non Del5q | ||
| MR (4) | 0 | 2 |
| No MR (14) | 6 | 10 |
| Total (18) | 6 | 12 |
| Hematologic Improvement - Erythroid (n) . | miR143 increased (n) . | miR145 increased (n) . |
|---|---|---|
| Del5q | ||
| MR (7) | 4 | 5 |
| No MR (1) | 1 | 0 |
| Total (8) | 5 | 5 |
| Non Del5q | ||
| MR (4) | 0 | 2 |
| No MR (14) | 6 | 10 |
| Total (18) | 6 | 12 |
Nevill:Celgene: Honoraria. Karsan:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal