Abstract
Abstract 1178
Effectively cryopreserving hematopietic peripheral blood progenitor cells (HPC(A)) until time of transplant is critical for successful autologous stem cell transplant. It is important to demonstrate long-term HPC(A) viability and recovery and also to identify patient characteristics that may influence this. We evaluated HPC(A) products cryopreserved over 16 year period. We analyzed potential correlations between total nucleated cell (TNC) and CD34+ recovery and viability and patient age, initial collection, and impact of stem cell mobilization regimen.
Samples were obtained from expired patients, chosen based on storage duration but randomly selected in regard to patient and product characteristics; 23 samples were obtained from 18 patients. Five patients had 2 samples each; for duplicate samples, data were averaged (collection dates and thaw dates were similar). All HPC(A) products were frozen in autologous plasma and 10% DMSO, then stored in liquid phase or vapor phase nitrogen. Products were uniformly thawed and washed to remove DMSO. Analysis included 7-Amino-actinomycin D viability, TNC and CD34+ cell counts and recovery. Some samples had post-thaw CD34+ cell counts that exceeded pre-thaw count; for these, the recovery was set to 100%. Spearman rank correlations were used to analyze association between the different parameters. Wilcoxon rank sum test and analysis of covariance were used to compare patient groups.
Median patient age at time of stem cell collection was 54 (range 2–75) years. A majority of patients had lymphoma (61%) or multiple myeloma (22%). Most (56%) were mobilized using G-CSF+VP16, 33% with G-CSF alone, and 11% other. Samples were cryopreserved for a median of 8 years (range 1–16). Median (range) for total CD34+ × 106 collected was 547 (43-5845). Median (range) for CD34+/kg × 106 was 8 (1-59). Median (range) count/bag for TNC × 108 was 144 (37-285) and for CD34+ × 106 was 105 (8-805). Median (range) recovery and viability were 85% (32-213%), and 70% (46-85%) respectively for TNC; and 97% (16-359%) and 80% (61-98%), respectively for CD34+ cells.
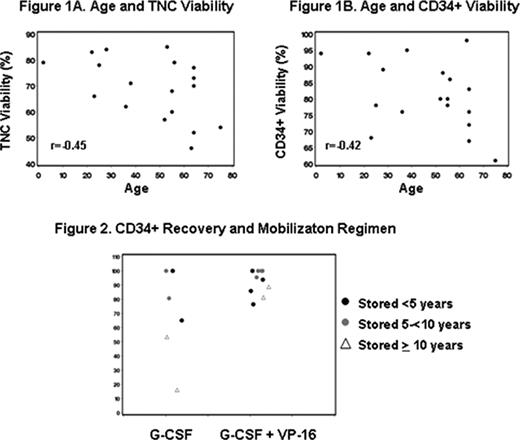
Pre- and post- thaw TNC and CD34+ counts were both highly correlated (r=0.95, p<.0001 in both cases – figures 1a and b). There was no significant association between TNC recovery and viability (r=-0.07, p=.79) or between CD34+ cell recovery and viability (r=-0.21, p=.41). Storage duration did not impact CD34+ recovery or viability (r=-0.01, p=.98 and -0.18, p=.47, respectively); or TNC viability (r=-0.13, p=.62). There was, however a significant positive correlation between the storage duration and TNC recovery (r=0.81, p<.0001).
Age did not significantly impact TNC or CD34+ recovery (r=-0.05, p=0.86, and r=-0.26, p=0.29, respectively); however there was suggestion of negative impact on viability (r=-0.45, p=0.06 and r=-0.42, p=0.08, respectively, figures 1a and 1b). Overall, there did not appear to be a correlation between the initial TNC count/bag or total CD34+ cell dose on recovery or viability (r=0.29, r=-0.22, r=0.18, r=-0.38, respectively; all p>0.12). However, when patients were stratified according to total collection >25×106 CD34+ cells/kg or ≤25 × 106 CD34+ cells/kg, there was better %CD34+ cell count recovery between groups (p = 0.07, median recovery 81% for 13 patients with ≤25 × 106 CD34+ cells/kg and 100% for 5 patients with >25×106 CD34+ cells/kg). For mobilization regimen, there was no significant difference in TNC recovery or viability (p = 0.19 and p=0.76, respectively) or CD34+ viability (p = 0.53), however there was suggestion that CD34+ recovery was greater with G-CSF+VP16 (p=0.06, see figure 2), independent of storage time.
Products cryopreserved for 16 years retain acceptable recovery and viability. Unexpectedly, storage duration positively correlated with TNC recovery, and CD34+ cell recovery of >100% was noted in several samples. Reasons for this are unclear, but are likely related to changes in enumeration method or use of methods validated for counting fresh cells. Patient age was suggested to negatively impact post-thaw HPC(A) viability. Our data also suggested that the mobilization regimen or the CD34+ cell collection yield may affect the CD34+ cell recovery, possibly reflecting differences in graft characteristics. The number of patients included is relatively small and these findings warrant further studies for validation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal