Abstract
Abstract 1151
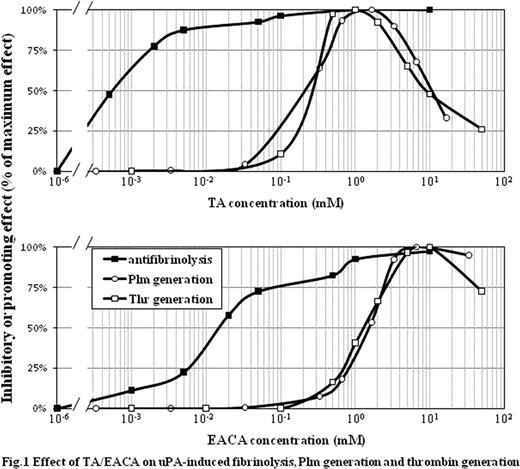
Tranexamic acid (TA) and epsilon-aminocaproic acid (EACA) of lysine analogs have been clinically used as antifibrinolytic agents. These hemostatic mechanism is that TA/EACA bind to lysine-binding sites (LBS) of plasmin (Plm)/plasminogen (Plg) and competitively prevents Plm/Plg from binding to fibrin(ogen), resulting in inhibition of Plm-induced fibrin(ogen) degradation. TA/EACA cause a conformational change of (Glu-)Plg by LBS binding, however, resulting in paradoxical promotion of Plg activation by Plg activators (PA). It has been known in vitro that TA/EACA promote Plm generation simultaneously with inhibiting fibrinolysis, but clinical effects are poor understood. We have recently demonstrated that Plm possessed the procoagulant activity by catalytic proteolysis of factor (F)VIII, FV as well as FXII. In this study, we examined whether TA/EACA affected on the coagulation system through elevation of PA-induced Plm generation. In rotation thromboelastometry (ROTEM), the addition of urokinase (uPA, 80 IU/ml) to whole blood diminished the maximum clot firmness, indicative of hyperfibrinolysis. Furthermore, chromogenic assay for Plm-hydrolytic activity and calibrated automated thrombography (CAT) revealed that the addition of uPA elevated Plm activity and peak level of thrombin generation, respectively, in normal plasma. These findings supported that uPA promoted Plm generation, resulting in enhancement of fibrinolysis and procoagulant activity. Various concentrations of TA/EACA were added into whole blood or plasma prior to reactions with uPA (Fig.1). Fibrinolytic effects of uPA obtained in ROTEM were inhibited by TA/EACA dose-dependently (IC50; TA/EACA ∼0.5 micro M/∼1.5 micro M), similar to previous reports. However, uPA (20 IU/ml) -induced Plm activity obtained in Plm-hydrolytic activity increased in the presence of TA/EACA by ∼6-fold (EC50; TA/EACA ∼0.2 mM/∼1.5 mM), followed by decreasing at higher concentrations. Interestingly, the effect of TA/EACA on uPA-induced procoagulant activity observed as elevation of peak thrombin in CAT was biphasic pattern, similar to that on Plm activity in Plm-hydrolytic activity, i.e. peak thrombin was elevated by ∼2-fold by TA/EACA (EC50; TA/EACA ∼0.3 mM/∼1.5 mM), and after reaching maximum (TA/EACA ∼1 mM/∼10 mM), it decreased. Effects of TA/EACA on Plm generation and thrombin generation were both diminished by aprotinin, a potent Plm inhibitor, indicating that the procoagulant effect interacted closely with Plm generation. Since α2-antiplasmin (AP) neutralizes Plm in plasma, excess of Plm unlikely exerts the procoagulant activity. Since AP binds to Plm via LBS, however, TA/EACA prevents AP from binding to Plm. We confirmed that TA/EACA protected Plm from AP binding (IC50; TA/EACA ∼1 mM/∼10 mM) in purified systems. Furthermore, in the presence of uPA in plasma, FV and FVIII activities were immediately elevated, followed by slow decrease. FVII activity increased gradually by ∼1.5-fold. TA/EACA did not inhibit the effects of uPA on the coagulation factors, but rather accelerated. Taken together, we demonstrated a novel hemostatic mechanism that TA/EACA exerted the procoagulant activity by LBS binding of Plg/Plm; i.e. 1) promoting uPA-induced Plm generation, 2) inhibiting Plm binding to fibrin(ogen) (increasing free Plm), 3) inhibiting neutralization of free Plm by AP, 4) conserving Plm action to several coagulation factors (FV, FVII, FVIII). This mechanism might provide a clarification of clinical effects of TA/EACA including why some severe hemophilia A patients were successfully treated with EACA alone (Ghosh et al. Haemophilia. 2004;10:58).
Ogiwara:Baxter Hemophilia Scientific Research and Education Fund in Japan 2009: Research Funding. Nogami:Bayer hemophilia award program 2009: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal