Abstract
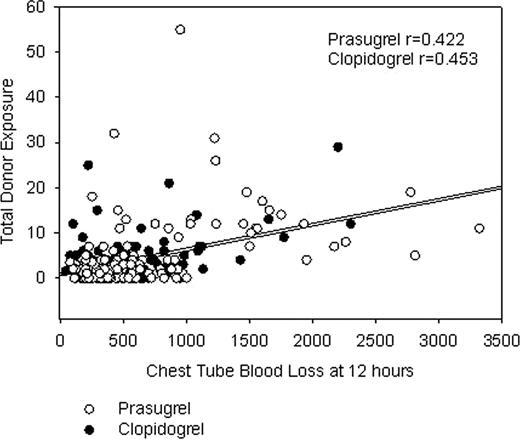
Coronary artery bypass grafting (CABG)-related bleeding and associated transfusion support are concerns with dual antiplatelet therapy. The amount and the type of blood products transfused may be influenced by patient characteristics, procedural characteristics, acquired hemostatic defects, regional standard of care, and the amount of perioperative blood loss. Withdrawal of clopidogrel 5 days prior or prasugrel 7 days prior to CABG is suggested by the respective product labels to reduce the potential for CABG-related bleeding. Accordingly, a cohort of 422 patients undergoing isolated CABG in TRITON-TIMI 38 (PRASugrel n=208; CLOPidogrel n=214) was analyzed retrospectively to characterize the risk adjusted (using predicted risk of transfusion - Magovern, et al. Ann Thorac Surg 1996;61:27-32) difference in transfusion requirements between prasugrel and clopidogrel. Seventy-six patients who never received study drug or who received open label thienopyridine treatment prior to CABG were excluded. Packed red blood cells (PRBC) were the most frequently used blood product while platelet transfusions were relatively infrequent and regionally specific. No difference was noted in mean predicted transfusion risk score (TRS) between cohorts (PRAS 2.13 vs. CLOP 2.31; p=0.53; two-sided p value per ANOVA). TRS was associated with the number of units of PRBC transfused (p < 0.0001). Overall, there were significantly higher mean 12 hour chest tube loss (655 ±580 ml vs. 503± 378 ml; Kruskal-Wallis p=0.050) and platelet transfusion rates (18% vs. 9.8%; Pearson's chi-square p=.033) with PRAS. However, there were no statistically significant differences in PRBC transfusion units (mean 2.1±3.0 PRAS vs. 1.7±2.2 CLOP; Kruskal-Wallis p=0.44) or total donor exposure units (PRBC + whole blood + platelets + cryoprecipitate + fresh frozen plasma) (4.4±7.6 PRAS vs. 3.0±4.5 CLOP; Kruskal-Wallis p=0.46), although all of the analyses presented are underpowered. Total donor exposures were correlated with chest tube blood loss at 12 hours and the correlations were similar for both cohorts (PRAS, r=0.42; CLOP, r=0.45) (Figure). Transfusion requirements and blood loss by days from last dose categories are presented in the Table. Fifty-four (54)%, 22%, and 24% of patients went to CABG surgery ≤5 days, 6–7 days, and >7 days from the last dose of study drug, respectively (days from last dose to CABG unknown for 0.6% of patients). A significantly higher number of platelet units were used postoperatively in the PRAS patients going to surgery ≤5 days after withdrawal of drug. There is no suggestion of excess blood loss or excess of total units of blood transfusions after 7 days for PRAS or CLOP. In an analysis adjusted for the predicted risk of mortality (using Society of Thoracic Surgeons mortality score), total donor exposures were not associated with increased mortality risk [all-cause death within 30 days following CABG (odds ratio, 1.05 (0.97- 1.13) p=0.25; logistic regression analysis]. In addition, PRAS was independently associated with a reduction in mortality (PRAS, 1.2%; CLOP, 6.9%; p=0.027). In summary, the amount of blood products transfused is significantly influenced by patient and surgical characteristics, as well as by agents that can inhibit the hemostatic system. However, despite increased blood loss and use of greater numbers of platelets in a higher percentage of patients within the PRAS cohort, use of PRAS was associated with improved outcomes in the isolated CABG cohort of TRITON-TIMI 38.
total donor exposure = PRBC + whole blood + platelets + cryoprecipitate
total donor exposure = PRBC + whole blood + platelets + cryoprecipitate
| . | . | 0–5 days . | 6–7 days . | >7 days . | |||
|---|---|---|---|---|---|---|---|
| Pras Mean N=88 . | Clop Mean N=99 . | Pras Mean N=34 . | Clop Mean N=41 . | Pras Mean N=50 . | Clop Mean N=32 . | ||
| Chest tube blood loss (ml) | 679 (618) | 534 (391) | 815 (667) | 463 (367) | 509 (405) | 464 (366) | |
| Intra-Operative | PRBC | 0.45 (1.0) | 0.71 (1.11) | 0.61 (1.69) | 0.44 (0.97) | 0.28 (0.62) | 0.76 (1.91) |
| Platelets | 0.33 (1.2) | 0.22 (1.13) | 0 (0) | 0.05 (0.32) | 0.23 (1.46) | 0.29 (1.19) | |
| Post–Operative | PRBC | 1.96 (3.38) | 1.17 (1.69) | 1.82 (2.44) | 0.92 (1.60) | 1.06 (1.69) | 0.87 (1.25) |
| Platelets | 0.95 (2.84)* | 0.25 (1.14) | 0.18 (0.53) | 0.08 (0.48) | 0.15 (0.87) | 0.23 (1.09) | |
| Total | PRBC | 2.39 (3.50) | 1.88 (2.22) | 2.42 (2.89) | 1.36 (1.83) | 1.33 (1.86) | 1.60 (2.70) |
| Platelets | 1.25 (3.25)* | 0.47 (1.57) | 0.18 (0.53) | 0.13 (0.57) | 0.38 (1.67) | 0.52 (2.25) | |
| Total donor exposure | 5.71 (9.45) | 3.29 (3.91) | 4.11 (5.64) | 2.26 (4.73) | 2.44 (3.80) | 3.05 (5.98) | |
| . | . | 0–5 days . | 6–7 days . | >7 days . | |||
|---|---|---|---|---|---|---|---|
| Pras Mean N=88 . | Clop Mean N=99 . | Pras Mean N=34 . | Clop Mean N=41 . | Pras Mean N=50 . | Clop Mean N=32 . | ||
| Chest tube blood loss (ml) | 679 (618) | 534 (391) | 815 (667) | 463 (367) | 509 (405) | 464 (366) | |
| Intra-Operative | PRBC | 0.45 (1.0) | 0.71 (1.11) | 0.61 (1.69) | 0.44 (0.97) | 0.28 (0.62) | 0.76 (1.91) |
| Platelets | 0.33 (1.2) | 0.22 (1.13) | 0 (0) | 0.05 (0.32) | 0.23 (1.46) | 0.29 (1.19) | |
| Post–Operative | PRBC | 1.96 (3.38) | 1.17 (1.69) | 1.82 (2.44) | 0.92 (1.60) | 1.06 (1.69) | 0.87 (1.25) |
| Platelets | 0.95 (2.84)* | 0.25 (1.14) | 0.18 (0.53) | 0.08 (0.48) | 0.15 (0.87) | 0.23 (1.09) | |
| Total | PRBC | 2.39 (3.50) | 1.88 (2.22) | 2.42 (2.89) | 1.36 (1.83) | 1.33 (1.86) | 1.60 (2.70) |
| Platelets | 1.25 (3.25)* | 0.47 (1.57) | 0.18 (0.53) | 0.13 (0.57) | 0.38 (1.67) | 0.52 (2.25) | |
| Total donor exposure | 5.71 (9.45) | 3.29 (3.91) | 4.11 (5.64) | 2.26 (4.73) | 2.44 (3.80) | 3.05 (5.98) | |
P<0.05 (Kruskal-Wallis test).
All Values M ± SD.
Goodnough:Eli Lilly and Company: Consultancy. Despotis:Eli Lilly Company: Consultancy. Levy:Eli Lilly Company: Consultancy, Research Funding. Short:Eli Lilly & Company: Employment, Equity Ownership. Weerakkody:Eli Lilly & Company: Employment, Equity Ownership. Lenarz:Eli Lilly & Company: Employment, Equity Ownership.
This icon denotes an abstract that is clinically relevant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal