Abstract
The bone morphogenic protein antagonist gremlin is expressed during embryonic development and under different pathologic conditions, including cancer. Gremlin is a proangiogenic protein belonging to the cystine-knot superfamily that includes transforming growth factor-β proteins and the angiogenic vascular endothelial growth factors (VEGFs). Here, we demonstrate that gremlin binds VEGF receptor-2 (VEGFR2), the main transducer of VEGF-mediated angiogenic signals, in a bone morphogenic protein–independent manner. Similar to VEGF-A, gremlin activates VEGFR2 in endothelial cells, leading to VEGFR2-dependent angiogenic responses in vitro and in vivo. Gremlin thus represents a novel proangiogenic VEGFR2 agonist distinct from the VEGF family ligands with implications in vascular development, angiogenesis-dependent diseases, and tumor neovascularization.
Introduction
The bone morphogenic protein (BMP) antagonist gremlin1 induces angiogenesis in a BMP-independent manner by binding to as-yet-unidentified endothelial cell (EC) membrane receptors and activating multiple tyrosine kinase–dependent intracellular signaling pathways in ECs.2,3 Gremlin is produced by human tumors4,5 and is expressed by fibroblast growth factor-2 (FGF2)–activated ECs and tumor endothelium.2 Thus, gremlin may play paracrine/autocrine roles in tumor neovascularization. The identification of the EC receptors activated by gremlin has so far been unsuccessful.
Vascular endothelial growth factor receptor-2 (VEGFR2) is the major proangiogenic tyrosine kinase receptor expressed by ECs and is activated by different members of the vascular endothelial growth factor (VEGF) family.6 Both gremlin and VEGFs belong to the cystine-knot protein superfamily,7 suggesting possible structural and/or functional similarities among these proangiogenic factors. On this basis, we investigated the capacity of gremlin to interact with and activate VEGFR2. The results demonstrate that gremlin binds and activates VEGFR2, leading to VEGFR2-dependent angiogenic responses in vitro and in vivo.
Methods
Ligand-receptor interaction assays
Interaction of VEGF-A and gremlin (R&D Systems) with the immobilized extracellular domain of VEGFR2 (sVEGFR2; Calbiochem) was analyzed by surface plasmon resonance (BIAcore Inc) and by competitive enzyme-linked immunosorbent assay (ELISA). VEGFR2 interaction on the EC surface was characterized by cross-linking experiments, whereas VEGFR2 dimerization was assessed by fluorescence resonance energy transfer analysis.
In vitro angiogenic assays
Motility and 3-dimensional gel invasion assays were performed on human, murine, and bovine ECs.3 When indicated, ECs were stably transfected with a pcDNA3.1 expression vector harboring the mouse VEGFR2 complementary DNA.
In vivo angiogenic assays
Alginate beads (5 μL) containing gremlin (100 ng per embryo) were grafted on the chorioallantoic membrane (CAM) of fertilized chicken eggs at day 11. After 72 hours, new blood vessels converging toward the implant were counted.
Details of experimental procedures and analysis are provided in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results and discussion
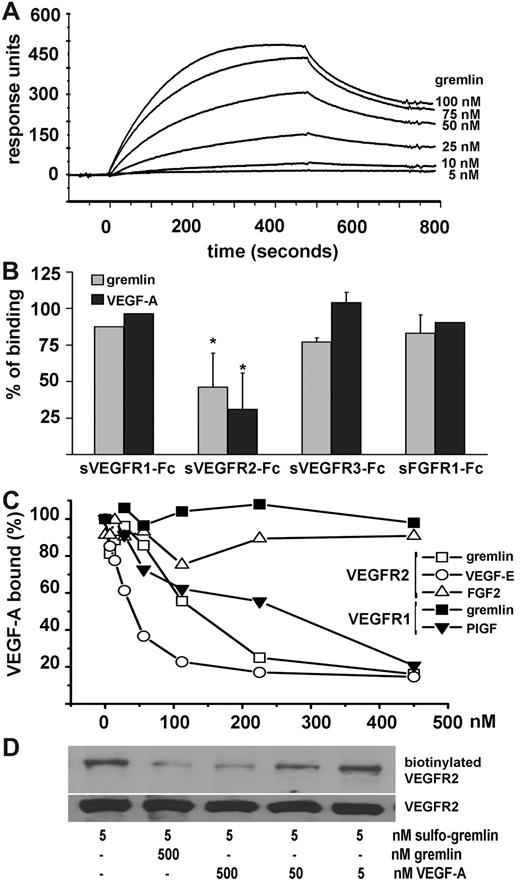
Surface plasmon resonance analysis was used to assess the ability of gremlin to bind the extracellular domain of VEGFR2. As shown in Figure 1A, gremlin binds sVEGFR2 immobilized on a BIAcore sensor chip (Kd = 47 ± 15 nM). The interaction is inhibited by an excess of soluble sVEGFR2-Fc but not by sVEGFR1-Fc, sVEGFR3-Fc, or sFGFR1-Fc (Figure 1B). Similar to VEGF-E, gremlin inhibits the binding of VEGF-A to immobilized sVEGFR2-Fc in competitive ELISA but, unlike the VEGFR1-specific agonist placenta growth factor, it does not affect VEGF-A interaction with sVEGFR1-Fc (Figure 1C). To assess whether gremlin/VEGFR2 interaction occurs also under physiologic conditions, we incubated human umbilical vein ECs (HUVECs) with gremlin conjugated with a bifunctional photoactivable biotin-label transfer cross-linker (sulfo-gremlin). After photoactivation of EC-bound sulfo-gremlin, VEGFR2 immunocomplexes were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions to transfer the biotin moiety to the interacting receptor, followed by detection with streptavidin–horseradish peroxidase. As shown in Figure 1D, sulfo-gremlin forms a 250 kDa biotin–VEGFR2 complex when cross-linked to the HUVEC surface, VEGFR2 biotinylation being inhibited by an excess of unlabeled gremlin or VEGF-A. Taken together, these data demonstrate the capacity of gremlin to bind VEGFR2 and to compete with VEGF-A for receptor interaction.
Gremlin binds VEGFR2. (A) sVEGFR2D1-7 (Calbiochem) was immobilized at approximately .083 pmol/mm2 to a CM5 sensorchip (BIAcore) that was previously activated with a mixture of 0.2M N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide hydrochloride and 0.05M N-hydroxysuccinimide (35 μL; flow rate: 10 μL/min). Increasing concentrations of gremlin were injected in HBS-EP buffer (BIAcore) for 4 minutes (sample volume: 40 μL; flow rate: 5 μL/min; dissociation time: 4 minutes). The response (in response units) was recorded as a function of time. An overlay plot is shown of all sensorgrams after subtraction of their respective control sensorgrams. Binding parameters, calculated by the nonlinear-curve–fitting software package BIAevaluation 3.2 (BIAcore Inc) applied to all sensorgrams simultaneously using a single-site model with drifting baseline, indicate that gremlin/VEGFR2 interaction occurs with Kd = 47 ± 15nM. Under the same experimental conditions, VEGF-A/VEGFR2 interaction occurs with Kd = 3 ± 1nM. (B) Gremlin (25nM) was injected over the sVEGFR2-coated sensor chip in the absence or in the presence of soluble sVEGFR1-Fc, sVEGFR2-Fc, sVEGFR3-Fc, or sFGFR1-Fc (all at 314nM). Binding data were plotted as percentage of maximal bound analyte (recorded at the end of injection) and represent the mean of 2-3 independent experiments. (C) Ninety-six–well plates coated with 100 μL of 250 ng/mL sVEGFR1-Fc or sVEGFR2-Fc were incubated with VEGF-A (20 ng/mL dissolved in phosphate-buffered saline containing 0.1% BSA, 5.0mM (ethylenedinitrilo)tetraacetic acid, 0.004% Tween 20 in presence of different competitors and incubated for 1 hour at 37°C followed by 1-hour incubation at room temperature. Bound VEGF-A was detected with an anti–human VEGF monoclonal antibody (R&D Systems). Gremlin competes with VEGF-A for the binding to immobilized sVEGFR2-Fc (□) in a competitive ELISA for which VEGF-E (○) and FGF2 (▵) were used as positive and negative controls, respectively. At variance, gremlin did not compete with VEGF-A for the binding to immobilized sVEGFR1-Fc (■), whereas placenta growth factor (PGIF) (▾) was fully effective. (D) HUVECs were incubated with 5.0nM gremlin conjugated with the bifunctional photoactivable biotin-label transfer cross-linker Sulfo-SBED Biotin Label transfer reagent (Pierce) (sulfo-gremlin) in the absence or in the presence of a molar excess of unlabeled gremlin or VEGF-A. After ultraviolet irradiation, cell lysates (1.0 mg of protein) were immunoprecipitated with anti-VEGFR2 antibody (Santa Cruz Biotechnology), separated on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel under reducing conditions and probed with streptavidin–horseradish peroxidase to visualize the biotin–VEGFR2 complex. Uniform loading of the gel was confirmed by probing the membrane with the anti-VEGFR2 antibody.
Gremlin binds VEGFR2. (A) sVEGFR2D1-7 (Calbiochem) was immobilized at approximately .083 pmol/mm2 to a CM5 sensorchip (BIAcore) that was previously activated with a mixture of 0.2M N-ethyl-N′-(3-dimethylaminopropyl)-carbodiimide hydrochloride and 0.05M N-hydroxysuccinimide (35 μL; flow rate: 10 μL/min). Increasing concentrations of gremlin were injected in HBS-EP buffer (BIAcore) for 4 minutes (sample volume: 40 μL; flow rate: 5 μL/min; dissociation time: 4 minutes). The response (in response units) was recorded as a function of time. An overlay plot is shown of all sensorgrams after subtraction of their respective control sensorgrams. Binding parameters, calculated by the nonlinear-curve–fitting software package BIAevaluation 3.2 (BIAcore Inc) applied to all sensorgrams simultaneously using a single-site model with drifting baseline, indicate that gremlin/VEGFR2 interaction occurs with Kd = 47 ± 15nM. Under the same experimental conditions, VEGF-A/VEGFR2 interaction occurs with Kd = 3 ± 1nM. (B) Gremlin (25nM) was injected over the sVEGFR2-coated sensor chip in the absence or in the presence of soluble sVEGFR1-Fc, sVEGFR2-Fc, sVEGFR3-Fc, or sFGFR1-Fc (all at 314nM). Binding data were plotted as percentage of maximal bound analyte (recorded at the end of injection) and represent the mean of 2-3 independent experiments. (C) Ninety-six–well plates coated with 100 μL of 250 ng/mL sVEGFR1-Fc or sVEGFR2-Fc were incubated with VEGF-A (20 ng/mL dissolved in phosphate-buffered saline containing 0.1% BSA, 5.0mM (ethylenedinitrilo)tetraacetic acid, 0.004% Tween 20 in presence of different competitors and incubated for 1 hour at 37°C followed by 1-hour incubation at room temperature. Bound VEGF-A was detected with an anti–human VEGF monoclonal antibody (R&D Systems). Gremlin competes with VEGF-A for the binding to immobilized sVEGFR2-Fc (□) in a competitive ELISA for which VEGF-E (○) and FGF2 (▵) were used as positive and negative controls, respectively. At variance, gremlin did not compete with VEGF-A for the binding to immobilized sVEGFR1-Fc (■), whereas placenta growth factor (PGIF) (▾) was fully effective. (D) HUVECs were incubated with 5.0nM gremlin conjugated with the bifunctional photoactivable biotin-label transfer cross-linker Sulfo-SBED Biotin Label transfer reagent (Pierce) (sulfo-gremlin) in the absence or in the presence of a molar excess of unlabeled gremlin or VEGF-A. After ultraviolet irradiation, cell lysates (1.0 mg of protein) were immunoprecipitated with anti-VEGFR2 antibody (Santa Cruz Biotechnology), separated on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel under reducing conditions and probed with streptavidin–horseradish peroxidase to visualize the biotin–VEGFR2 complex. Uniform loading of the gel was confirmed by probing the membrane with the anti-VEGFR2 antibody.
We next investigated whether gremlin induces VEGFR2 autophosphorylation in ECs.6 In HUVECs, gremlin induced a dose-dependent phosphorylation of tyrosine phosphorylation sites Y1175 and Y951 of VEGFR2 (supplemental Figure 1a) with kinetics similar to VEGF-A (Figure 2A-B). VEGFR2 activation is also abrogated by the VEGFR2 tyrosine kinase inhibitor SU54168 both in gremlin and VEGF-A–activated ECs (Figure 2C). Accordingly, VEGFR2 phosphorylation, abrogated by SU5416, was observed in fetal bovine aortic VEGFR2-GM7373 ECs stimulated by gremlin or VEGF-A after immunostaining with anti–phospho-VEGFR2 (pY1175) antibodies (supplemental Figure 2). VEGFR2 engagement leads to a rapid internalization of the activated receptor.9 Indeed, confocal analysis shows that both gremlin and VEGF-A induce VEGFR2 phosphorylation and internalization of the activated receptor in HUVECs (Figure 2D). In addition, fluorescence resonance energy transfer analysis of bovine aortic ECs cotransfected with enhanced yellow fluorescent protein–tagged and enhanced cyan fluorescent protein–tagged VEGFR2 highlighted the internalization of VEGFR2 dimers in the early endosomal compartment9 after stimulation with gremlin or VEGF-A but not with FGF2 (supplemental Figure 3). Thus, in keeping with its ability to induce focal adhesion kinase, mitogen-activated protein kinase extracellular signal-regulated kinase1/2, and transcription factor nuclear factor κB activation,2,3 gremlin activates VEGFR2 similar to VEGF-A. Tyrosine phosphorylation of cellular proteins (supplemental Figure 1a), chemotactic migratory response and small GTPase Rac activation (supplemental Figure 4), as well as the formation of 3-dimensional EC sprouts (Figure 2E) are similar in HUVECs stimulated by either gremlin or VEGF-A. These activities are blocked by SU5416 or by receptor-binding competitors such as neutralizing anti-VEGFR2 antibody10 or the cyclic peptide cyclo-VEGI (Calbiochem).11
VEGFR2 activation mediates the proangiogenic activity of gremlin in vitro and in vivo. Serum-starved HUVECs were stimulated for 0-30 minutes with 50 ng/mL gremlin (A) or 30 ng/mL VEGF-A (B) or with 50 ng/mL gremlin or 30 ng/mL VEGF-A for 15 minutes in the absence or in the presence of 5.0μM SU5416 (C). At the end of incubation, 50 μg of cell extracts were probed by Western blotting with a monoclonal anti–phospho-VEGFR2 antibody (pY1175; Cell Signaling Technology). Uniform loading of gels was confirmed by incubation of the membranes with anti–focal adhesion kinase (FAK) antibodies (Santa Cruz Biotechnology). Capture ELISA assay ruled out a significant VEGF-A contamination of the recombinant gremlin preparations (≤ 0.1 pg of VEGF-A per nanogram of protein). Accordingly, gremlin-induced VEGFR2 autophosphorylation in ECs was not affected by coincubation with a neutralizing anti–VEGF-A antibody (data not shown). (D) Serum-starved HUVECs were stimulated for 0 or 5 minutes with 50 ng/mL gremlin or 30 ng/mL VEGF-A and immunostained with rabbit monoclonal anti–phospho-VEGFR2 antibody (pY1175) followed by Alexa Fluor 488 anti–rabbit IgG (Invitrogen). Samples were analyzed using a Zeiss LSM510Meta confocal microscope equipped with Plan-Apochromat 63×/1.4 numerical aperture oil objective and LSM 510 Meta Software Version 3.5 (Carl Zeiss). Note the presence of the phosphorylated receptor both on HUVEC membrane and in submembrane vesicles in stimulated cells. (E) HUVEC spheroids prepared in medium supplemented with carboxymethylcellulose were embedded in type I collagen gel and treated with vehicle or 30 ng/mL gremlin or VEGF-A in the absence or in the presence of 3.0μM cyclo-VEGI. Formation of radially growing cell sprouts was observed during the next 48 hours.3 Sprouts were photographed at 40× magnification with an IX51 inverted microscope equipped with a 4×/0.10 numerical aperture objective and a Camedia C-4040 digital camera (Olympus). Data are expressed as total number of sprouts measured in 50 spheroids. *Statistically different from the stimulus in the absence of any inhibitor (P < .01, Student t test). (F) One-millimeter human umbilical artery rings were embedded in fibrin gel and incubated with 50 ng/mL gremlin, 30 ng/mL VEGF-A, or vehicle alone in absence or in the presence of 5.0μM SU5416 or 20nM VEGFR2 kinase inhibitor I (VEGFR2I). After 3 days, EC sprouts, morphologically distinguishable from scattering fibroblasts/smooth muscle cells, were counted at 100× magnification with an IX51 inverted microscope equipped with a Plan A chromatic phase contrast 10×/0.25PhP numerical aperture objective (Olympus). *Statistically different from gremlin or VEGF-A–treated rings (P < .01, Student t test). (G) Alginate beads containing vehicle, 100 ng of gremlin, or VEGF-A were implanted on the top of chick embryo CAMs at day 11 of development. When indicated, pellets also contained 150 ng of sVEGFR2 or 5.0μM SU5416. After 72 hours, newly formed blood vessels converging toward the implant were counted in ovo at 5× magnification using a STEMI SR stereomicroscope equipped with an objective f equal to 100 mm with adapter ring 475070 (Carl Zeiss). Data are expressed as mean ± SEM (n = 6-8). *Statistically different from the stimulus in the absence of any inhibitor (P < .01, Student t test).
VEGFR2 activation mediates the proangiogenic activity of gremlin in vitro and in vivo. Serum-starved HUVECs were stimulated for 0-30 minutes with 50 ng/mL gremlin (A) or 30 ng/mL VEGF-A (B) or with 50 ng/mL gremlin or 30 ng/mL VEGF-A for 15 minutes in the absence or in the presence of 5.0μM SU5416 (C). At the end of incubation, 50 μg of cell extracts were probed by Western blotting with a monoclonal anti–phospho-VEGFR2 antibody (pY1175; Cell Signaling Technology). Uniform loading of gels was confirmed by incubation of the membranes with anti–focal adhesion kinase (FAK) antibodies (Santa Cruz Biotechnology). Capture ELISA assay ruled out a significant VEGF-A contamination of the recombinant gremlin preparations (≤ 0.1 pg of VEGF-A per nanogram of protein). Accordingly, gremlin-induced VEGFR2 autophosphorylation in ECs was not affected by coincubation with a neutralizing anti–VEGF-A antibody (data not shown). (D) Serum-starved HUVECs were stimulated for 0 or 5 minutes with 50 ng/mL gremlin or 30 ng/mL VEGF-A and immunostained with rabbit monoclonal anti–phospho-VEGFR2 antibody (pY1175) followed by Alexa Fluor 488 anti–rabbit IgG (Invitrogen). Samples were analyzed using a Zeiss LSM510Meta confocal microscope equipped with Plan-Apochromat 63×/1.4 numerical aperture oil objective and LSM 510 Meta Software Version 3.5 (Carl Zeiss). Note the presence of the phosphorylated receptor both on HUVEC membrane and in submembrane vesicles in stimulated cells. (E) HUVEC spheroids prepared in medium supplemented with carboxymethylcellulose were embedded in type I collagen gel and treated with vehicle or 30 ng/mL gremlin or VEGF-A in the absence or in the presence of 3.0μM cyclo-VEGI. Formation of radially growing cell sprouts was observed during the next 48 hours.3 Sprouts were photographed at 40× magnification with an IX51 inverted microscope equipped with a 4×/0.10 numerical aperture objective and a Camedia C-4040 digital camera (Olympus). Data are expressed as total number of sprouts measured in 50 spheroids. *Statistically different from the stimulus in the absence of any inhibitor (P < .01, Student t test). (F) One-millimeter human umbilical artery rings were embedded in fibrin gel and incubated with 50 ng/mL gremlin, 30 ng/mL VEGF-A, or vehicle alone in absence or in the presence of 5.0μM SU5416 or 20nM VEGFR2 kinase inhibitor I (VEGFR2I). After 3 days, EC sprouts, morphologically distinguishable from scattering fibroblasts/smooth muscle cells, were counted at 100× magnification with an IX51 inverted microscope equipped with a Plan A chromatic phase contrast 10×/0.25PhP numerical aperture objective (Olympus). *Statistically different from gremlin or VEGF-A–treated rings (P < .01, Student t test). (G) Alginate beads containing vehicle, 100 ng of gremlin, or VEGF-A were implanted on the top of chick embryo CAMs at day 11 of development. When indicated, pellets also contained 150 ng of sVEGFR2 or 5.0μM SU5416. After 72 hours, newly formed blood vessels converging toward the implant were counted in ovo at 5× magnification using a STEMI SR stereomicroscope equipped with an objective f equal to 100 mm with adapter ring 475070 (Carl Zeiss). Data are expressed as mean ± SEM (n = 6-8). *Statistically different from the stimulus in the absence of any inhibitor (P < .01, Student t test).
To further assess the role of VEGFR2 in mediating the proangiogenic activity of gremlin, we transfected murine aortic ECs (MAECs) expressing low levels of VEGFR2 with a murine VEGFR2 complementary DNA giving rise to VEGFR2-MAECs. The motogenic activity exerted by gremlin or VEGF-A is dramatically up-regulated in these cells compared with parental MAECs. Again, the activity of both motogens is inhibited by SU5416 and cyclo-VEGI (supplemental Figure 5).
In keeping with these in vitro observations, the angiogenic activity exerted by gremlin and VEGF-A ex vivo in the human umbilical artery ring EC sprouting assay and in vivo in the chick embryo CAM assay2,3 is significantly inhibited by SU5416, the VEGFR2 inhibitor I (VEGFR2-KI),12 or by competition with an excess of free sVEGFR2 (Figure 2F-G; supplemental Figures 6-7). In all the assays, VEGFR2 inhibitors do not affect the activity of FGF2 (data not shown).
Here, we demonstrate that the angiogenic activity of gremlin is mediated by VEGFR2. Most importantly, BMP2 does not prevent gremlin from binding and activating VEGFR2 (supplemental Figure 8). Similarly, BMP4 does not affect the angiogenic activity of gremlin and its interaction with ECs.2 We therefore propose that distinct domains of gremlin may mediate the interaction with VEGFR2 or BMPs, respectively.
The capacity of gremlin to bind BMPs and to inhibit their interaction with the cognate transforming growth factor-β family receptors is thought to play a role in embryonic development13 and in human diseases.14 However, BMP-independent mechanism(s) may also be involved in gremlin signaling.2,15 Our data reveal the previously unrecognized capacity of gremlin to specifically bind to and promote activation of the major proangiogenic receptor VEGFR2 in a BMP-independent manner. Thus, gremlin may exert both BMP-dependent and BMP-independent functions in different physio-pathologic conditions by inhibiting BMP-mediated transforming growth factor-β receptor activation or by a direct activation of VEGF receptor signaling, respectively. These findings extend the number of proangiogenic VEGFR2 ligands to a member of the cystine-knot BMP antagonists distinct from VEGFs.16 Gremlin is expressed by parenchymal and stromal cells in human tumors.2,4,5 Our observations may provide novel insights for the understanding of the biology of vascular development and of angiogenesis-dependent diseases, including cancer.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from Istituto Superiore di Sanità (Oncotechnological Program), Ministero dell'Istruzione, Università e Ricerca (Centro di Eccellenza per l'Innovazione Diagnostica e Terapeutica; Cofin projects), Associazione Italiana per la Ricerca sul Cancro (AIRC), Fondazione Berlucchi, and Fondazione Cariplo (grant 2008-2264 and NOBEL Project) to M.P., from AIRC (MFAG 9161 grant) to S.M., and from Swiss National Science Foundation (grant 3100A-116507) and Oncosuisse (grant OC2 01200-08-2007) to K.B.-H.
Authorship
Contribution: S.M., C.R., E.M., L.Z., K.B.-H., and M.P. designed research; S.M., C.R., E.M., V.S., D.L., and L.Z. performed research; S.M., C.R., E.M., K.B.-H., and M.P. analyzed data; and S.M., C.R., K.B.-H., and M.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco Presta, General Pathology, Department of Biomedical Sciences and Biotechnology, University of Brescia, Viale Europa 11, 25123 Brescia, Italy; e-mail: presta@med.unibs.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal