Abstract
In vivo targeting of C-type lectin receptors is an effective strategy for increasing antigen uptake and presentation by dendritic cells (DCs). To induce efficient immune response, glycosylated tumor-associated Tn antigens were used to target DCs through binding to macrophage galactose-type lectin (MGL). The capacity of Tn-glycosylated antigens—and the multiple antigenic glycopeptide Tn3 therapeutic candidate vaccine—to target mouse and human MGL+ DCs are demonstrated, especially regarding dermal DCs. In mice, MGL+ CD103− dermal DCs efficiently captured and processed glycosylated Tn antigen in vivo, inducing a potent major histocompatibility complex (MHC) class II–restricted T-cell response. Intradermal immunization with Tn-glycopeptides induced high levels of Th2 cytokines—even in the presence of unmethylated cytosine-phosphate-guanosine—and was associated with increased expansion of the germinal center B-cell population. Therefore, MGL acts as an efficient endocytic antigen receptor on dermal DCs in vivo, able to prime Tn-specific T- and B-cell responses. Moreover, even in the absence of adjuvant, immunization with this glycosidic Tn-based vaccine induced high levels of anti-Tn antibody responses, recognizing human tumor cells. In vivo DC-targeting strategies, based on Tn-MGL interactions, constitute a promising strategy for enhancing antigen presentation and inducing potent antibody response.
Introduction
Recent strategies for improving prophylactic and therapeutic vaccines have focused on the efficient delivery of antigen to dendritic cells (DCs).1,2 DCs acquire and process antigen and migrate to the lymphoid organs where they present antigen to specific T cells, thereby inducing primary T- and B-cell responses. DCs recognize pathogens through various pattern-recognition receptors, including toll-like receptors and C-type lectin receptors (CLRs). CLRs bind to conserved carbohydrate-based structures on pathogens, thereby inducing innate immune responses.3 CLRs recognize glycoproteins from bacteria,4 fungi,5 and viruses,6 contributing to the carbohydrate-mediated endocytosis of such pathogens.7 CLRs also specifically recognize endogenous ligands and perform various immune and homoeostatic functions.8
Strategies based on CLR on DC targeting in vivo represent an effective means to enhance antigen uptake and presentation by DCs.9 Moreover, such in vivo direct approaches may overcome laborious ex vivo DC manipulations and reinjection into cancer patients to induce antitumor T-cell responses.2 Two approaches have been used to target CLR, the first of which is based on coupling antigen to CLR-specific antibodies. Indeed, targeting antigen to DEC-20510 and DNGR111 has enhanced the uptake and presentation of the antigen by MHC class I and II pathways, inducing efficient and long-lasting immune response. An alternative strategy is based on antigen binding to CLR ligands, such as mannose or mannan, which specifically target the mannose receptor on immature DCs and enhanced the efficiency of antigen presentation by MHC class I and II routes.9 However, although these sugars bind to the mannose receptor, they are not only recognized by this CLR and, therefore, probably also target the antigen to multiple lectins with overlapping binding specificities.12
Macrophage galactose-type lectin (MGL; CD301), a C-type lectin first described on macrophages,13 is a type II transmembrane protein that recognizes terminal N-acetylgalactosamine (GalNAc) residues in a calcium-dependent manner. It was first described as an asialoglycoprotein receptor on immature human DCs, on which it acts as an endocytic receptor, specifically capturing antireceptor monoclonal antibody (mAb) and delivering them to the endosomal pathway.14 The MGL ligands have been exhaustively studied, unlike those of other CLRs.15 MGL displays a remarkable specificity for the Tn antigen,16 which consists of serine- or threonine-linked terminal GalNAc residues. The Tn antigen is expressed on human carcinoma–associated mucin variants15 and is considered a tumor biomarker. In humans, MGL is preferentially expressed by DCs and macrophages—the most potent antigen-presenting cells (APCs) in the immune system—although it is absent from monocytes, plasmacytoid DCs, and lymphocytes.15 It is also expressed by interstitial DCs in the small intestine as well as interstitial-type DCs in the skin.14 In mice, 2 very similar isoforms, mMGL1 and mMGL2, have been identified with similar expression profiles.15 mMGL isoforms are also expressed on inflammatory macrophages17 and marrow-derived DCs from immature bone.18 mMGL has been detected in the dermis, lymph nodes, and in the lamina propia of the large intestine.19 Of these 2 lectins, mMGL2 specifically recognizes GalNAc residues, including the Tn antigen.17
Given the remarkable specificity of MGL for the tumor-associated Tn antigen, and its presence in potent APCs such as DCs, we designed a new vaccine strategy based on targeting glycosylated Tn antigen to DCs through MGL binding. Two vaccine candidates carrying the Tn antigen were evaluated: (1) multiple antigenic glycopeptide (MAG):Tn, based on a dendrimeric lysine core with 4 arms linked to a peptide backbone containing a CD4+ T-cell epitope20 and (2) mucin-like Tn-glycoproteins produced by in vitro glycosylation using ppGalNAc-Ts.21 The MAG:Tn vaccine candidate designed for human vaccination was demonstrated to induce in nonhuman primates strong anti-Tn antibodies specifically recognizing and mediating killing of Tn-expressing human tumor cells.22 Our present data demonstrate that intradermal immunization based on the DC-targeting strategy via carbohydrate-lectin interactions results in the efficient and selective delivery of antigen to MGL+ dermal DCs. This delivery, in turn, leads to strong T-cell activation and expansion of the germinal center (GC) B-cell population, together with the induction of high levels of specific anti-Tn antibodies.
Methods
Mice
Six- to 10-week-old female BALB/c or C57BL/6 mice (CER JANVIER and Charles River) were kept in the Institut Pasteur animal house in specific pathogen–free conditions. Water and food were supplied ad libitum. Experiments involving animals were conducted according to institutional guidelines for animal care.
Chemical and enzymatic synthesis of dendrimeric glycopeptides and MUC6:Tn glycoproteins
MAG antigens are based on a dendrimeric lysine core with 4 arms linked to a peptide backbone that contains a CD4+ T-cell epitope (PV, TT, or MalE) with trimeric or hexameric saccharide Tn residues at the N-terminal end of the peptide. MAG:Tn glycopeptides contain either 3 Tn (MAG:Tn3-TT, MAG:Tn3-PV, and MAG:Tn3-MalE) or 6 Tn (MAG:Tn6-PV) residues per arm. MAG glycopeptides and nonglycosylated multiple antigenic peptides (MAP; MAP-TT, MAP-PV, and MAP-MalE) were produced chemically by solid-phase synthesis, as previously described.20 The TT and PV peptide sequences correspond to the CD4+ T-cell epitopes of tetanus toxin (QYIKANSKFIGITEL) and poliovirus (PV; KLFAVWKITYKDT), respectively. The MalE peptide sequence corresponds to the CD4+ T-cell epitope of maltose-binding protein from Escherichia coli (NGKLIAYPIAVEALS).
Tn glycoproteins were obtained by enzymatic GalNAc transfer using polypeptide-GalNAc transferases on an 86-amino acid recombinant protein carrying the sequence of a half-tandem repeat of human MUC6, as previously described.21 Alexa647 labeling of peptides and glycopeptides was completed (FluoProbes Protein Labeling Kit; Interchim).
mAb for flow cytometry
Antibodies against human MGL/CD301 (125A10.03) were purchased from DENDRITICS. Antibodies against CD205 (NLDC-145) and mouse MGL/CD301 (ER-MP23) were obtained from AbD Serotec. Antibodies against anti–human CD14 (61D3) and CD1a (HI149) and against mouse B220 (RA3-6B2), CD11b (M1/70), CD11c (N418), CD3 (145-2C11), CD49b (DX5), CD103 (2E7), Langerin/CD207 (eBioL31), EpCAM/CD326 (G8.8), FcϵRI (MAR-1), mPDCA-1 (eBio927), as well as T cell– and B cell–activating antigen (GL-7), and rat IgG2a isotype control were obtained from eBioscience. Antibodies against CD4 (RM4-5), CD8α (53-6.7), CD40 (HM40-3), CD45.2 (104), CD45RA (14.8), and I-A/I-E (2G9) were obtained from BD Biosciences. FITC-labeled PNA was obtained from Sigma-Aldrich and used at a concentration of 1 mg/mL.
Preparation of human DCs and Tn antigen–binding assay
Approval was received from the Ethical Approval Committee of Institut Pasteur to obtain adult blood and umbilical cord blood samples from consenting mothers in accordance with the Declaration of Helsinki. Human monocytes were purified using anti-CD14 Microbeads (Miltenyi Biotec) from adult peripheral blood mononuclear cell. Monocyte-derived DCs (MoDCs) were generated by culturing monocytes with 100 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF) and 100 ng/mL IL-4 for 5 days. CD34+ hematopoietic progenitors purified from cord blood mononuclear cell were cultured with 100 ng/mL GM-CSF, 100 ng/mL TNF-α, and 25 ng/mL stem cell factor (SCF) for 5 days. CD1a+ and CD14+ cells were sorted by FACS-Aria II (BD Biosciences) and were further cultured with GM-CSF and TNF-α for another 6-8 days to obtain Langerhans cells (LCs) and dermal-like DCs, respectively.23 For antigen-binding assay, human monocytes, MoDCs, LCs, or dermal-like DCs were incubated with dye-labeled antigen for 10 minutes at 4°C, washed twice, and analyzed by FACS. For inhibition assays, cells were incubated with 10mM of ethylenediaminetetraacetic acid (EDTA).
Preparation of mouse DCs
Bone marrow–derived DCs (BMDCs) were generated using bone marrow precursors from C57BL/6 or BALB/C mice in medium containing 50mM of 2-mercaptoethanol and 1% of a GM-CSF–containing supernatant. Cells were recovered on day 6 or 7 and usually contained 60%-70% CD11c+ cells. Alternatively, bone marrow cells were incubated with FLt3-L for 9 days to generate plasmacytoid DC (BMpDC).
Mouse dermal DC ear explant cultures
Dermal DCs were isolated from ear explant cultures from untreated mice.24 The cartilage-containing side was removed from mouse ears. Epidermis was removed after dispase I incubation (Roche Applied Science). The dermis was incubated for 48 hours in 12-well plates containing complete medium supplemented with 0.1 μg/well 6Ckine (R&D Systems). The cells migrating out from the skin were analyzed by flow cytometry.
Antigen tracking
We injected 10 nmol MAP-PV, MAG:Tn3-PV, MAG:Tn6-PV, MUC6, or MUC6:Tn labeled with Alexa488 or Alexa647 intradermally into the ears of the mice. Skin draining lymph nodes (DLN) and spleen collected 18 hours later were incubated with collagenase type IV (400 U/mL) and 50 mg/mL DNaseI (Roche Applied Science).
In vitro Tn-glycopeptide internalization assay
Flow cytometry was used to analyze in vitro internalization of MAG:Tn-PV and MUC6:Tn glycopeptides. BMDCs or dermal DCs were incubated with Alexa647-labeled antigen for 1 hour at 37°C to assess uptake or at 4°C to assess binding. Cells were then washed twice and analyzed by FACS. For inhibition assays, cells and glycopeptides were incubated in complete medium supplemented with 10mM of EDTA or 10 μg/mL anti-MGL mAb (ERMP23; Cedarlane Labs) for 1 hour.
Antigen presentation assay
T-cell hybridomas were generated in our laboratory. The 45G10 T-cell hybridoma is specific for the PV peptide KLFAVWKITYKDT and is I-Ed–restricted.25 The CRMC3 T-cell hybridoma is specific for the MalE protein from E coli (NGKLIAYPIAVEALS) and is I-Ab–restricted.26 The 1D10 T-cell hybridoma is specific for the MUC6 peptide SSTSLVTPSTHTVIT and is I-Ab–restricted. For inhibition assays, BMDCs or interstitial DCs were incubated with Tn-glycopeptides in complete medium supplemented with 5mM of EDTA, anti-MGL antibody (ERMP23 clone), or anti-Tn mAb (2F9; 10 μg/mL) for 4 hours. After washing, PV-specific or MalE-specific T-cell hybridomas were added and the suspension was incubated for 24 hours. We then quantified IL-2 either by enzyme-linked immunosorbent assay (ELISA) or using the IL-2–dependent CTLL cell line.
DC purification and ex vivo antigen presentation assay
BALB/c mice were injected intradermally into the ear with phosphate-buffered saline (PBS) alone or with 10 nmol MAP-PV, MAG:Tn3-PV, or MAG:Tn6-PV. After 18 hours, skin DLN were removed and incubated with 400 U/mL collagenase type IV and 50 mg/mL DNaseI. Single-cell suspensions were prepared and DCs were sorted magnetically using anti-CD11c Microbeads (Miltenyi Biotec). DC subsets were further purified, as indicated, by flow cytometry. For ex vivo antigen presentation assays, DC suspensions obtained from mice immunized with different glycopeptides were serially diluted and cultured with 105 T-cell hybridomas. Supernatants were harvested, frozen, and their IL-2 content was determined by ELISA.
T-cell proliferation assays and cytokine production
We injected 10 nmol MAP-PV, MAG:Tn3-PV, or MAG:Tn6-PV in PBS intradermally into the ears of the mice together with 10 μg of unmethylated cytosine-phosphate-guanosine (CpG) or PBS alone. After 10 days, draining lymph nodes and spleen cell suspensions were stimulated in vitro with serial dilutions of MAG:Tn3-PV or PV peptide for 72 hours. We assessed the levels of secreted cytokines in culture supernatants (Luminex Corporation), as previously described.27
Induction of anti-Tn antibodies and ELISA
BALB/c mice were immunized intradermally with 10 nmol MAP:PV or MAG:Tn3-PV with 10 μg of CpG in PBS or with 25 μg of anti-CD40 antibody. Control mice received CpG or anti-CD40 antibodies alone. Mouse sera were collected either 14 or 21 days after injection and were tested for anti-Tn antibodies by ELISA, as previously described.20 Titers are expressed as the arithmetic mean ± SD of the log10 titers.
Results
Synthetic MAG:Tn glycopeptide binds to MGL+ human DCs
The human MGL lectin is a candidate receptor for the targeting of DCs through recognition of the Tn antigen.14,15 We previously developed a therapeutic anticancer vaccine candidate, MAG:Tn3-TT. This synthetic 15 kD Tn-glycopeptide is based on a dendrimeric lysine core with 4 arms linked to a peptide backbone containing a promiscuous CD4+ T-cell epitope from tetanus toxin with a trimeric Tn cluster at the N-terminal end of the peptide.22 MAG:Tn3-TT induces strong tumor-specific anti-Tn antibodies in 2 nonhuman primate species.22 In the present study, we aimed to investigate whether Tn-glycopeptides could target DCs through recognition by MGL.
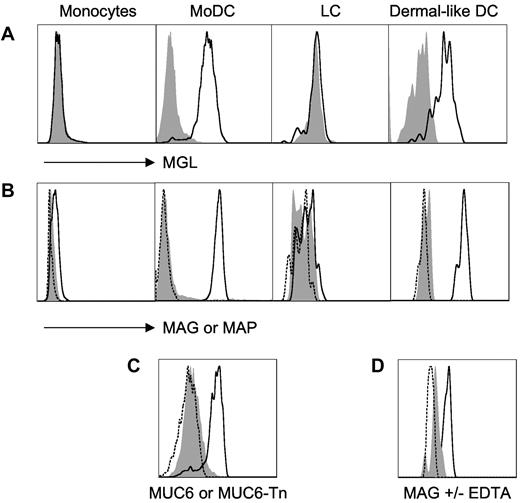
Human monocyte–derived DCs as well as dermal-like DCs—but not monocytes or LCs—strongly expressed MGL (Figure 1A). Next, we tested the capacity of human DCs to bind Alexa647-labeled MAG:Tn3-TT glycopeptide vs nonglycosylated control MAP:TT peptide (Figure 1B). Both MGL+ MoDCs and dermal-like DCs efficiently bound the MAG, but not the MAP, whereas LCs did not.
Tn-glycosylated antigens target human dermal DCs. Representative results from 3-5 experiments are shown in panels A, B, and D; panel C corresponds to a single experiment. Unstained cells are shown as dashed line. (A) Human monocytes, MoDCs, CD34+-derived Langerhans cells, and dermal-like DCs were tested for macrophage galactose-type lectin expression using macrophage galactose-type lectin antibodies (bold line) or isotype control antibodies (shaded gray; FACS-Aria II, BD Biosciences). (B) Alternatively, prior to FACS analysis, these cell types were incubated at 4°C, as indicated, with Alexa488-labeled glycosylated multiple antigenic glycopeptide (MAG):Tn3-TT (bold line), or unglycosylated multiple antigenic peptides (MAP):TT (shaded gray), or medium (dashed line; FluoProbes Protein Labeling Kit, Interchim). (C) Monocyte-derived DCs were incubated at 4°C with Alexa647-labeled MUC6-Tn (bold line), MUC6 (shaded gray), or medium (dashed line). (D) Incubation of MAG:Tn3-TT with dermal-like DCs was performed in the presence (shaded gray) or absence (bold line) of ethylenediaminetetraacetic acid to evaluate divalent cation-dependency binding to dermal DCs.
Tn-glycosylated antigens target human dermal DCs. Representative results from 3-5 experiments are shown in panels A, B, and D; panel C corresponds to a single experiment. Unstained cells are shown as dashed line. (A) Human monocytes, MoDCs, CD34+-derived Langerhans cells, and dermal-like DCs were tested for macrophage galactose-type lectin expression using macrophage galactose-type lectin antibodies (bold line) or isotype control antibodies (shaded gray; FACS-Aria II, BD Biosciences). (B) Alternatively, prior to FACS analysis, these cell types were incubated at 4°C, as indicated, with Alexa488-labeled glycosylated multiple antigenic glycopeptide (MAG):Tn3-TT (bold line), or unglycosylated multiple antigenic peptides (MAP):TT (shaded gray), or medium (dashed line; FluoProbes Protein Labeling Kit, Interchim). (C) Monocyte-derived DCs were incubated at 4°C with Alexa647-labeled MUC6-Tn (bold line), MUC6 (shaded gray), or medium (dashed line). (D) Incubation of MAG:Tn3-TT with dermal-like DCs was performed in the presence (shaded gray) or absence (bold line) of ethylenediaminetetraacetic acid to evaluate divalent cation-dependency binding to dermal DCs.
We also analyzed binding to MoDCs of MUC6:Tn obtained by in vitro glycosylation of an MUC6-recombinant protein with recombinant ppGalNAc-T1.21 These enzymatically synthesized molecules induce the production of Tn-specific antibodies that recognize human tumor cells.21 The Tn-glycosylated form of the MUC6 mucin efficiently bound to MGL+ DCs whereas the unglycosylated MUC6 did not (Figure 1C). Binding of MAG:Tn3-TT to dermal-like DCs was inhibited by EDTA in accord with the Ca2+ dependency of carbohydrate recognition by MGL (Figure 1D). Altogether, these results clearly show the capacity of Tn-glycosylated antigens—and, in particular, the MAG:Tn3-TT therapeutic candidate vaccine—to target human MGL+ DCs, especially dermal-like DCs.
In vivo enhanced uptake and presentation of glycosylated Tn antigen by its targeting to MGL+ dermal DCs
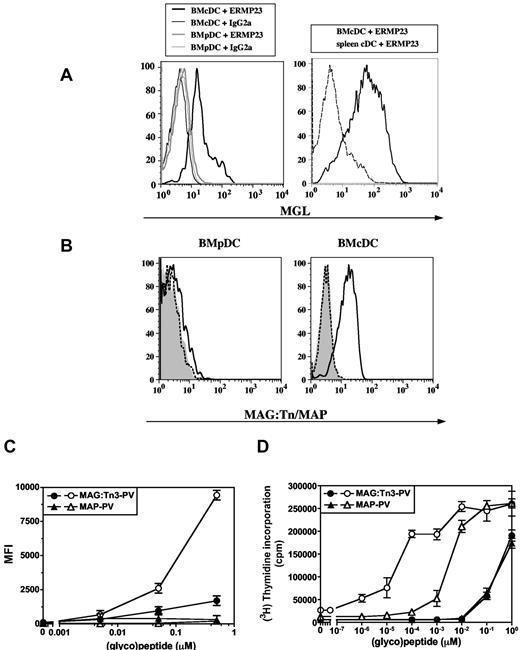
To further investigate whether MGL targeting could be an efficient in vivo antigen-delivery strategy, we used dendrimeric Tn-glycopeptides containing mouse CD4+ T-cell epitope from PV or MalE protein (ie, MAG:Tn3-PV and MAG:Tn3-MalE, respectively). We first analyzed MGL expression by murine conventional DC (BMcDC) and BMpDC derived from bone marrow cultures in vitro as well as by splenic cDC (Figure 2A). Results demonstrated that only BMcDC, but not splenic cDC or BMpDC, express MGL. We then analyzed the targeting of MAG:Tn3-PV glycopeptide to MGL+ BMcDC or to MGL− BMpDC. We subjected BMDCs to 1 hour pulses with various doses of the Alexa647-labeled MAG:Tn3-PV glycopeptide or the nonglycosylated control MAP-PV peptide. A strong capture and endocytosis by BMcDC of MAG:Tn3-PV glycopeptide, but not of MAP-PV, was observed under these conditions, indicating an effect of Tn glycosylation on antigen uptake. Moreover, MGL+ BMcDC were much more efficient in capturing MAG:Tn3-PV glycopeptide than MGL− BMpDC (Figure 2B-C). When incubated with a T-cell hybridoma specific for the PV epitope, MAG:Tn3-PV–loaded BMcDC displayed a strong capacity to stimulate specific T cells, compared with cells loaded with the nonglycosylated control peptide MAP-PV. Only a weak—and similar—level of stimulation was obtained with MGL− BMpDC loaded with either MAG:Tn3-PV or MAP-PV (Figure 2D). Accordingly, the MHC class II presentation by BMDCs of a mucin protein carrying the Tn antigen (MUC6:Tn[MCF7]), mimicking those produced by cancer cells, to a mucin-specific CD4+ T-cell hybridoma was much stronger than that observed with the nonglycosylated mucin (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Inhibition assays confirmed the involvement of Tn in the recognition of these glycoconjugates by MGL on BMDCs. Indeed, the presentation of MAG:Tn3-MalE to the MalE-specific CD4+ T-cell hybridoma was strongly inhibited by incubating BMDCs with the Tn-glycopeptide in the presence of the anti-MGL mAb ERMP23 (supplemental Figure 1B) or an anti-Tn mAb (supplemental Figure 1C), whereas these mAbs had no effect on the presentation of the nonglycosylated MAP-PV. Finally, comparative analysis of the presentation of various glycopeptides carrying the same PV CD4+ T-cell epitope confirmed that enhanced presentation of MAG:Tn3-PV by DCs was Tn-mediated (supplemental Figure 2).
Multiple antigenic glycopeptide (MAG):Tn-poliovirus (PV) glycopeptide uptake and presentation is enhanced in macrophage galactose-type lectin (MGL) positive bone marrow–derived dendritic cells (BMDCs). (A) BMDCs or splenic conventional DCs (cDCs) from BALB/c mice were incubated with an anti-MGL monoclonal antibody (ERMP23 clone) or with an isotype IgG2a antibody, followed by a PE-conjugated anti–rat IgG2a antibody. MGL expression was analyzed by flow cytometry on CD11c+, CD45RA−, and CD11b+ gated cells for murine cDC (BMcDC); on CD11c+ for splenic cDC; and on CD11c+, CD45RA+, PDCA1+, and CD11b− gated cells for plasmacytoid DC (BMpDC). (B) To assess the binding capacity of BMDCs to antigens, BMpDCs and murine conventional DCs (BMcDCs) were incubated at 4°C with Alexa488-labeled glycosylated MAG:Tn3-TT (bold line), unglycosylated MAP:TT (shaded gray), or medium (dashed line). (C) MAG:Tn3-PV and nonglycosylated MAP-PV Alexa647-labeled antigen were incubated with BMDCs. We monitored internalization by flow cytometry on BMpDC (closed symbols) or BMcDC (open symbols) gated cells. Data are expressed as the mean ± SD of triplicates. Representative results from 3 experiments are presented. (D) The Tn-glycopeptide and nonglycosylated peptide were incubated with purified BMcDC (open symbols) or BMpDC (closed symbols) and the PV-specific T-cell hybridoma 45G10 T-cell hybridoma. IL-2 responses were assessed using the IL-2–dependent CTLL cell line. Data are expressed as the mean ± SD of triplicates. Representative results from 3 experiments are shown.
Multiple antigenic glycopeptide (MAG):Tn-poliovirus (PV) glycopeptide uptake and presentation is enhanced in macrophage galactose-type lectin (MGL) positive bone marrow–derived dendritic cells (BMDCs). (A) BMDCs or splenic conventional DCs (cDCs) from BALB/c mice were incubated with an anti-MGL monoclonal antibody (ERMP23 clone) or with an isotype IgG2a antibody, followed by a PE-conjugated anti–rat IgG2a antibody. MGL expression was analyzed by flow cytometry on CD11c+, CD45RA−, and CD11b+ gated cells for murine cDC (BMcDC); on CD11c+ for splenic cDC; and on CD11c+, CD45RA+, PDCA1+, and CD11b− gated cells for plasmacytoid DC (BMpDC). (B) To assess the binding capacity of BMDCs to antigens, BMpDCs and murine conventional DCs (BMcDCs) were incubated at 4°C with Alexa488-labeled glycosylated MAG:Tn3-TT (bold line), unglycosylated MAP:TT (shaded gray), or medium (dashed line). (C) MAG:Tn3-PV and nonglycosylated MAP-PV Alexa647-labeled antigen were incubated with BMDCs. We monitored internalization by flow cytometry on BMpDC (closed symbols) or BMcDC (open symbols) gated cells. Data are expressed as the mean ± SD of triplicates. Representative results from 3 experiments are presented. (D) The Tn-glycopeptide and nonglycosylated peptide were incubated with purified BMcDC (open symbols) or BMpDC (closed symbols) and the PV-specific T-cell hybridoma 45G10 T-cell hybridoma. IL-2 responses were assessed using the IL-2–dependent CTLL cell line. Data are expressed as the mean ± SD of triplicates. Representative results from 3 experiments are shown.
Thus, Tn-bearing antigens are efficiently targeted to the MGL receptor on BMDCs for potent MHC class II presentation to T cells in vitro.
Glycosylated Tn antigen targets dermal interstitial DCs in vivo
We next evaluated the potential of in vivo antigen delivery to DCs by targeting MGL with Tn glycopeptides. First, we analyzed MGL expression on DCs freshly isolated from mouse skin. In contrast to splenic DCs (Figure 2A), interstitial DCs from dermis isolated from mouse ears expressed MGL (Figure 3A), confirming previous findings.19 The dermis contains 3 main populations of DCs with different functions: migrating LCs from the epidermis as well as dermal CD103+ and CD103− DCs. These cells can be distinguished on the basis of their expression profiles for CD11b, epithelial-cell adhesion molecule (EpCAM), Langerin, and CD103.28 MGL was strongly expressed by CD103− dermal DCs (CD11c+ MHCII+ EpCam− CD11b+ CD103−), but not by other DC subsets (Figure 3A).
Tn-glycosylated antigens target dermal dendritic cells (dDCs) in vivo. (A) Analysis of macrophage galactose-type lectin expression (bold line) in Langerhans cells (CD11c+ major histocompatibility complex [MHC]) II+ epithelial-cell adhesion molecule positive [EpCam+] CD11b+ Langerin+ CD103−), CD103+ dDC (CD11c+ MHCII+ EpCam− CD11b−), and CD103− dDC (CD11c+ MHCII+ EpCam− CD11b+ CD103−) from BALB/c mouse skin. Isotype control staining is shown as a gray histogram. (B) CD45+ CD11c+ cells from the dermis were incubated at 4°C or 37°C with Alexa647-labeled antigen glycosylated multiple antigenic glycopeptide (MAG):Tn3-poliovirus (PV; left panel), MUC6:Tn(MCF7) (right panel), or nonglycosylated controls MAP-PV and MUC6, respectively. Antigen binding and uptake were analyzed in the presence or absence of ethylenediaminetetraacetic acid, anti–macrophage galactose-type lectin monoclonal antibody (ERMP23), or control Ig2a (FACS-Aria II; BD Biosciences). (C) Interstitial DCs from dermis were incubated with glycosylated MAG:Tn3-PV or nonglycosylated multiple antigenic peptides-PV and cultured with a PV-specific T-cell hybridoma. IL-2 release by the T-cell hybridoma was analyzed in the 24-hour supernatant by enzyme-linked immunosorbent assay. (D) Alexa647- or (E) Alexa488-labeled MAG:Tn glycopeptides or nonglycosylated multiple antigenic peptides-PV were injected intradermally into the right ear of mice. (D) Eighteen hours later, antigen capture was analyzed by flow cytometry in CD45+ CD11c+ Langerin− cells present in the dermis, by comparison with antigen-free dermis of the left ear. (E) Alternatively, cells that have captured labeled antigen were directly analyzed from skin by gating on Langerhans cells (gray line), CD103+ (dashed line), and CD103− (bold line) dDCs. Mean fluorescence intensities (MFI) are shown in the boxes. Representative results from 3 experiments are shown.
Tn-glycosylated antigens target dermal dendritic cells (dDCs) in vivo. (A) Analysis of macrophage galactose-type lectin expression (bold line) in Langerhans cells (CD11c+ major histocompatibility complex [MHC]) II+ epithelial-cell adhesion molecule positive [EpCam+] CD11b+ Langerin+ CD103−), CD103+ dDC (CD11c+ MHCII+ EpCam− CD11b−), and CD103− dDC (CD11c+ MHCII+ EpCam− CD11b+ CD103−) from BALB/c mouse skin. Isotype control staining is shown as a gray histogram. (B) CD45+ CD11c+ cells from the dermis were incubated at 4°C or 37°C with Alexa647-labeled antigen glycosylated multiple antigenic glycopeptide (MAG):Tn3-poliovirus (PV; left panel), MUC6:Tn(MCF7) (right panel), or nonglycosylated controls MAP-PV and MUC6, respectively. Antigen binding and uptake were analyzed in the presence or absence of ethylenediaminetetraacetic acid, anti–macrophage galactose-type lectin monoclonal antibody (ERMP23), or control Ig2a (FACS-Aria II; BD Biosciences). (C) Interstitial DCs from dermis were incubated with glycosylated MAG:Tn3-PV or nonglycosylated multiple antigenic peptides-PV and cultured with a PV-specific T-cell hybridoma. IL-2 release by the T-cell hybridoma was analyzed in the 24-hour supernatant by enzyme-linked immunosorbent assay. (D) Alexa647- or (E) Alexa488-labeled MAG:Tn glycopeptides or nonglycosylated multiple antigenic peptides-PV were injected intradermally into the right ear of mice. (D) Eighteen hours later, antigen capture was analyzed by flow cytometry in CD45+ CD11c+ Langerin− cells present in the dermis, by comparison with antigen-free dermis of the left ear. (E) Alternatively, cells that have captured labeled antigen were directly analyzed from skin by gating on Langerhans cells (gray line), CD103+ (dashed line), and CD103− (bold line) dDCs. Mean fluorescence intensities (MFI) are shown in the boxes. Representative results from 3 experiments are shown.
We then analyzed antigen uptake by interstitial DCs isolated from the dermis by incubating these cells with fluorescent glycosylated Tn antigen or with nonglycosylated control antigen. Dermal DCs took up MAG:Tn3-PV and MUC6:Tn(MCF7) glycopeptides much more efficiently than nonglycosylated peptides in vitro (Figure 3B and supplemental Figure 3). Both the anti-MGL mAb ERMP23 and EDTA efficiently inhibited Tn-antigen uptake at 37°C (Figure 3B), demonstrating that binding was Tn-specific and mediated by MGL. Furthermore, when incubated with a PV-specific CD4+ T-cell hybridoma, interstitial DCs from the dermis presented MAG:Tn3-PV glycopeptide much more efficiently than nonglycosylated control MAP-PV peptide (Figure 3C). Thus, in vitro Tn antigen uptake by MGL on interstitial DCs enhances MHC class II presentation. Having demonstrated the MGL-dependent loading of dermal DCs in vitro with MAG:Tn3-PV, leading to antigen presentation to T cells, we assessed in vivo targeting of Tn-glycopeptides to DCs. We injected mice intradermally with fluorescent glycosylated or nonglycosylated Tn antigen and followed the uptake of these antigens by flow cytometry using DCs from the dermis recovered from cultured ear explants, 18 hours after injection. Unlike nonglycosylated MAP-PV, the MAG:Tn-PV glycopeptides, which were carrying 3 or 6 Tn, were efficiently captured by interstitial CD103− DCs (Figure 3D-E). Tn glycosylation is thus an efficient manner of targeting the MGL in vivo on interstitial DCs.
CD103− dermal DCs preferentially present glycosylated Tn antigen to T cells in lymph nodes
Skin DLN contain diverse DC subsets, including migratory and resident DCs. The migratory population consists of epidermis-derived LCs and dermis-derived CD103+ and CD103− DCs (supplemental Figure 4), all of which are critical for antigen trafficking from peripheral sites to the lymph nodes, allowing antigen presentation to T cells.29,30 We first analyzed the MGL expression profile in various DC subsets from skin DLN (Figure 4A). Consistent with the expression profile of skin DCs, only the CD103− DC subset migrating from the dermis expressed MGL. We then tracked DCs that captured glycosylated Tn antigen in the dermis after intradermal immunization. The 4 Tn antigens tested, MAG:Tn-PV glycopeptides, and MUC6-Tn glycoproteins were all efficiently captured by migratory DCs from skin DLN (Figure 4B). By contrast, no antigen-associated fluorescence was detected in skin DLN DCs from mice injected with nonglycosylated antigen or PBS, or in the non-DLN. Only the MGL+ CD103− dermal DC subset, corresponding to dermal DCs migrating from the skin, was targeted by the Tn antigen at the dermis and migrated to the DLN (Figure 4C).
CD103− dermal dendritic cells (dDCs) capture and transport glycosylated Tn antigen to the draining lymph nodes (DLN) for presentation to T cells. (A) Analysis of macrophage galactose-type lectin expression (bold line) in skin DLN DCs: CD8α+ DCs (CD11c+ major histocompatibility complex [MHC]) II+ CD11b− CD8α+), Langerhans cells (CD11c+ MHCII+ CD8α− Langerin+ CD103−), CD103+ dDC (CD11c+ MHCII+ CD8α− Langerin+ CD103+), and CD103− dDC (CD11c+ MHCII+ CD11b+ Langerin− CD103−). Isotype control staining is shown as a gray histogram. (B-C) PBS, 10 nmol Alexa647-labeled glycosylated Tn antigen, or nonglycosylated control antigen was injected intradermally into the mouse ear. After 24 hours, we analyzed skin DLN, non-DLN, or spleen total CD11c+ DCs (B) and DLN DC subsets migrating from the skin (C) for antigen capture by flow cytometry. (D-E) We injected mice intradermally, in both ears, with 10 nmol multiple antigenic glycopeptide:Tn3-poliovirus (PV), multiple antigenic glycopeptide:Tn6-PV, nonglycosylated multiple antigenic peptides-PV peptide, or PBS. Total CD11c+ DCs (D) and DLN DC subsets migrating from the skin (E) were purified and cultured with a PV-specific T-cell hybridoma 24 hours later. IL-2 release by the T-cell hybridoma was analyzed in the 24-hour supernatants by enzyme-linked immunosorbent assay. Data are expressed as mean ± SD of duplicates. Representative results from 2 experiments are shown.
CD103− dermal dendritic cells (dDCs) capture and transport glycosylated Tn antigen to the draining lymph nodes (DLN) for presentation to T cells. (A) Analysis of macrophage galactose-type lectin expression (bold line) in skin DLN DCs: CD8α+ DCs (CD11c+ major histocompatibility complex [MHC]) II+ CD11b− CD8α+), Langerhans cells (CD11c+ MHCII+ CD8α− Langerin+ CD103−), CD103+ dDC (CD11c+ MHCII+ CD8α− Langerin+ CD103+), and CD103− dDC (CD11c+ MHCII+ CD11b+ Langerin− CD103−). Isotype control staining is shown as a gray histogram. (B-C) PBS, 10 nmol Alexa647-labeled glycosylated Tn antigen, or nonglycosylated control antigen was injected intradermally into the mouse ear. After 24 hours, we analyzed skin DLN, non-DLN, or spleen total CD11c+ DCs (B) and DLN DC subsets migrating from the skin (C) for antigen capture by flow cytometry. (D-E) We injected mice intradermally, in both ears, with 10 nmol multiple antigenic glycopeptide:Tn3-poliovirus (PV), multiple antigenic glycopeptide:Tn6-PV, nonglycosylated multiple antigenic peptides-PV peptide, or PBS. Total CD11c+ DCs (D) and DLN DC subsets migrating from the skin (E) were purified and cultured with a PV-specific T-cell hybridoma 24 hours later. IL-2 release by the T-cell hybridoma was analyzed in the 24-hour supernatants by enzyme-linked immunosorbent assay. Data are expressed as mean ± SD of duplicates. Representative results from 2 experiments are shown.
We then investigated the role of dermal DCs in the presentation of glycosylated Tn antigen to T cells in the lymph nodes by isolating DCs from skin DLN 24 hours after MAG:Tn-PV intradermal administration and carrying out ex vivo antigen presentation assays with an MHC II–restricted PV-specific T-cell hybridoma. Both MAG:Tn-PV glycopeptides carrying different densities of Tn antigen, were efficiently presented to specific T cells by CD11c+ DCs purified from skin DLN, whereas MAP-PV peptide was not (Figure 4D). CD103− DCs from mice immunized intradermally with MAG:Tn3-PV was the only DLN-purified DC subset which efficiently stimulated the PV-specific T-cell hybridoma. Only a very weak antigen presentation was detected with LCs, CD103+ or CD8+ lymph node DC subsets from MAG:Tn3-PV immunized mice (Figure 4E) or with CD103− DCs from mice injected with MAP-PV.
Thus, Tn glycosylation efficiently targeted antigen to dermal DCs leading to MHC II presentation in the DLN.
In vivo targeting of dermal DCs with glycosylated Tn antigen induces a Th2-type response and high titers of anti-Tn antibodies
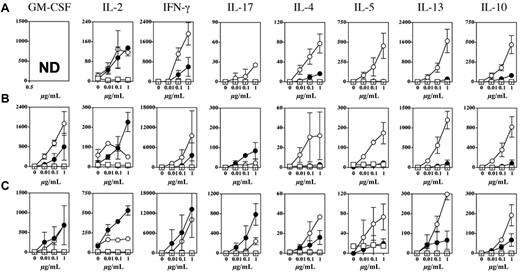
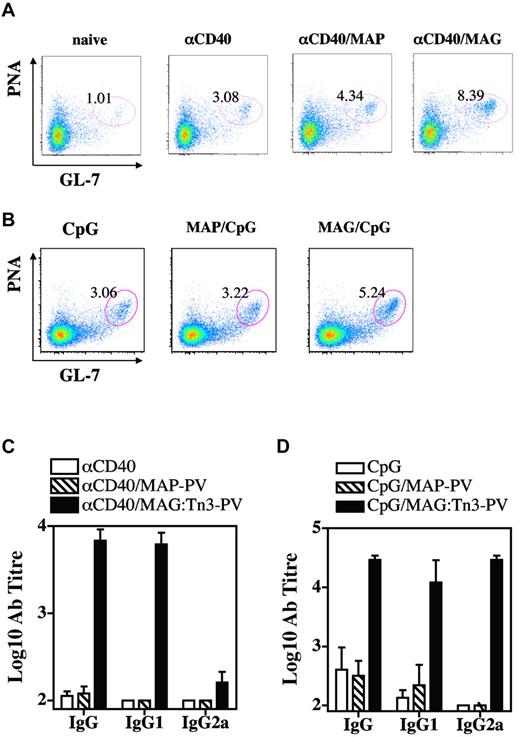
We then analyzed the type of T-cell response induced by MGL+ dermal DCs targeted by glycosylated Tn antigen. Mice were injected intradermally with glycosylated MAG:Tn3-PV or nonglycosylated MAP-PV Tn antigen, with or without CpG, and T cells from skin DLN or spleen were analyzed for cytokine production after in vitro stimulation with MAG:Tn3-PV (Figure 5). Glycosylated and nonglycosylated antigen induced comparable T-cell responses when assessed by measuring GM-CSF, IL-2, and IFN-γ production. However, in some cases, MAG:Tn3-PV induced slightly higher levels of GM-CSF and IFN-γ in skin DLN, whereas MAP-PV–primed splenocytes produced higher levels of IL-2. A very different situation was observed for the induction of Th2 cytokines. Indeed, high levels of IL-4, IL-5, IL-10, and IL-13 were detected after intradermal immunization of mice with glycosylated Tn antigen, but not with MAP-PV, in spleen and skin DLN. Even in the presence of CpG during vaccination, Tn antigen strongly induced production of Th2 cytokines (Figure 5B-C). Given the ability of MAG:Tn3-PV to prime CD4+ Th2 cells, we investigated whether these cells elicited B-cell responses. We assessed the induction and expansion of GC B cells in skin DLN from mice immunized intradermally with glycosylated MAG:Tn3-PV or nonglycosylated control MAP-PV in the presence of strong activation stimuli, such as anti-CD40 mAb (Figure 6A) or CpG (Figure 6B). MAG:Tn3-PV induced more GC PNA+ GL-7+ B cells31 than nonglycosylated MAP-PV control when injected with anti-CD40 mAb or CpG (Figure 6A-B). GC B cells generate memory B cells and long-lived plasma cells secreting high-affinity antigen-specific immunoglobulins.32 Thus, high levels of GC B cells induced by Tn-glycopeptide may reflect a strong induction of Tn-specific effector B cells. Therefore, we evaluated the ability of MAG:Tn3-PV to mount a Tn-specific antibody response when injected under these conditions (Figure 6C-D). After a single intradermal injection, high levels of IgG1 and IgG2a anti-Tn antibodies were induced by MAG:Tn3-PV injected with CpG. By contrast, only IgG1 anti-Tn antibodies were obtained when this glycopeptide was injected together with nonpolarizing anti-CD40 mAb. This efficient induction of anti-Tn antibodies was not due to a direct effect of MAG:Tn3-PV on B cells, as shown by the lack of in vitro activation of these lymphocytes by Tn-glycopeptide (supplemental Figure 5).
Antigen delivery to dermal dendritic cells by Tn-based targeting preferentially induces a Th2-like response. We injected BALB/c mice (2 or 3 per group) intradermally with PBS (□), glycosylated multiple antigenic glycopeptide:Tn3-poliovirus (PV; ○), nonglycosylated multiple antigenic peptides:PV (•), or (A) nonglycosylated multiple antigenic peptides:PV with 10 μg of unmethylated cytosine-phosphate-guanosine (B-C). Two weeks later, draining lymph node cells (A-B) or T cells purified from the spleen (C) were analyzed for antigen-specific T-cell responses after stimulation with multiple antigenic glycopeptide:Tn3-PV. Cytokine production was analyzed by the Luminex system (Luminex Corporation). Data are expressed as mean ± SD picograms per milliliter of duplicates. Representative results from 2-4 experiments are presented. ND indicates not done.
Antigen delivery to dermal dendritic cells by Tn-based targeting preferentially induces a Th2-like response. We injected BALB/c mice (2 or 3 per group) intradermally with PBS (□), glycosylated multiple antigenic glycopeptide:Tn3-poliovirus (PV; ○), nonglycosylated multiple antigenic peptides:PV (•), or (A) nonglycosylated multiple antigenic peptides:PV with 10 μg of unmethylated cytosine-phosphate-guanosine (B-C). Two weeks later, draining lymph node cells (A-B) or T cells purified from the spleen (C) were analyzed for antigen-specific T-cell responses after stimulation with multiple antigenic glycopeptide:Tn3-PV. Cytokine production was analyzed by the Luminex system (Luminex Corporation). Data are expressed as mean ± SD picograms per milliliter of duplicates. Representative results from 2-4 experiments are presented. ND indicates not done.
Tn-based targeting preferentially promotes B-cell and antibody responses. BALB/c mice (3 or 4 per group) were injected intradermally with 10 nmol of glycosylated multiple antigenic glycopeptide (MAG):Tn3-PV or unglycosylated multiple antigenic peptides PV peptide together with 25 μg of anti-CD40 antibody (A) or 10 μg of unmethylated cytosine-phosphate-guanosine (B). Control groups remained untreated or received αCD40 or unmethylated cytosine-phosphate-guanosine. Two to 3 weeks later, draining lymph node cells were analyzed for germinal center B cells by FACS (A and B). Serum samples were collected from immunized mice 2-3 weeks after intradermal immunization and anti-Tn antibody titers were determined (C-D). Data are expressed as the mean ± SD of 3 or 4 sera. Representative results from 2 experiments are shown.
Tn-based targeting preferentially promotes B-cell and antibody responses. BALB/c mice (3 or 4 per group) were injected intradermally with 10 nmol of glycosylated multiple antigenic glycopeptide (MAG):Tn3-PV or unglycosylated multiple antigenic peptides PV peptide together with 25 μg of anti-CD40 antibody (A) or 10 μg of unmethylated cytosine-phosphate-guanosine (B). Control groups remained untreated or received αCD40 or unmethylated cytosine-phosphate-guanosine. Two to 3 weeks later, draining lymph node cells were analyzed for germinal center B cells by FACS (A and B). Serum samples were collected from immunized mice 2-3 weeks after intradermal immunization and anti-Tn antibody titers were determined (C-D). Data are expressed as the mean ± SD of 3 or 4 sera. Representative results from 2 experiments are shown.
Immunization with MAG:Tn-PV in the absence of adjuvant induces potent antitumoral antibody response
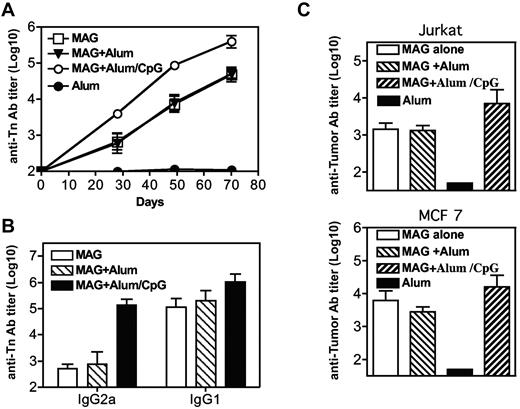
The immunogenicity of MAG:Tn3-PV was also analyzed after subcutaneous injections (at days 0, 21, 42, and 63), either alone or in the presence of alum or alum and CpG. As shown in Figure 7A and B, similar high IgG1, but low IgG2a, anti-Tn titers were observed after immunization in the absence of adjuvant or with alum. IgG1 titers were increased by the addition of CpG whereas IgG2a responses reached levels comparable with IgG1 titers. MAG:Tn3-PV–immunized mice developed strong antitumor responses, as evidenced by the recognition of Tn+ Jurkat and human breast adenocarcinoma MCF 7 cells (Figure 7C).
Immunization with multiple antigenic glycopeptide (MAG):Tn-poliovirus (PV)in the absence of adjuvant induces a potent antitumoral antibody response. BALB/c mice were immunized subcutaneously on days 0, 21, 42, and 63 with MAG:Tn3-PV, MAG:Tn3-PV with alum, or alum with unmethylated cytosine-phosphate-guanosine as adjuvant (4 mice per group). Control mice received only alum. (A) Mice were bled on days 20, 28, 49 and 70 to determine anti-Tn IgG antibody content in sera by enzyme-linked immunosorbent assay. Antibody isotypes were analyzed by enzyme-linked immunosorbent assay (B). Antitumor IgG titers were assessed at day 70 on Tn+ Jurkat and MCF 7 cells (C; FACS-Aria II, BD Biosciences). Data are expressed as mean ± SEM titers.
Immunization with multiple antigenic glycopeptide (MAG):Tn-poliovirus (PV)in the absence of adjuvant induces a potent antitumoral antibody response. BALB/c mice were immunized subcutaneously on days 0, 21, 42, and 63 with MAG:Tn3-PV, MAG:Tn3-PV with alum, or alum with unmethylated cytosine-phosphate-guanosine as adjuvant (4 mice per group). Control mice received only alum. (A) Mice were bled on days 20, 28, 49 and 70 to determine anti-Tn IgG antibody content in sera by enzyme-linked immunosorbent assay. Antibody isotypes were analyzed by enzyme-linked immunosorbent assay (B). Antitumor IgG titers were assessed at day 70 on Tn+ Jurkat and MCF 7 cells (C; FACS-Aria II, BD Biosciences). Data are expressed as mean ± SEM titers.
Basophils, which function as APCs and induce Th2-response activation,33 do not express MGL (supplemental Figure 6) and were therefore not involved in the induction of Th2 responses and anti-Tn antibodies by Tn-glycosylated antigen.
Overall, our findings demonstrate that the targeting of DCs in vivo with Tn glycopeptides results in preferential stimulation of CD4+ Th2 responses and the induction of high levels of antibodies specific for the tumor-associated Tn antigen, even in the absence of adjuvant.
Discussion
Targeting studies have revealed that the in vivo efficacy of DC vaccination depends on many factors, including the expression pattern and biologic properties of the targeted receptor and the maturation or activation status of DCs.2 In the present study, we showed that Tn-glycopeptides target interstitial DCs in vivo, activate CD4+ T cells, and induce a strong antitumoral Tn-specific antibody response, suggesting that the targeting of dermal DCs in humans with Tn-glycosylated peptides is a strategy of choice for future cancer immunotherapy. This hypothesis is supported by the observation that MAG:Tn glycopeptides protect mice against Tn+ tumor cells in prophylactic and therapeutic immunization protocols.20 Moreover, the MAG:Tn3-TT vaccine candidate designed for human vaccination and carrying human pan–human leukocyte antigen DR–restricted CD4+ T-cell epitopes was found to induce strong anti-Tn IgG antibodies that specifically recognized Tn-expressing human tumor cells in nonhuman primates.22 MAG:Tn-induced antibodies mediate the killing of tumor cells by complement-dependent cytotoxicity or by antibody-dependent cell-mediated toxicity, confirming that MAG:Tn molecules are effective for immunotherapeutic purposes in humans.22 We demonstrate here the ability of this vaccine candidate to specifically target human MGL+ DCs. Based on the MAG:Tn3-TT vaccine, we are currently organizing a clinical trial in breast cancer patients.
It has been previously shown in vitro that MGL on immature DCs can internalize Tn-containing molecules18 and deliver them for degradation into HLA compartments.34 We reported here functional studies regarding presentation of antigen endocytosed by MGL in vivo. Our results provide the first evidence that Tn-glycopeptides are more effectively captured and presented to CD4+ T cells by MGL+ DCs than nonglycosylated antigen. In agreement with our results, the involvement of murine MGL2 in glycosylated antigen uptake and presentation by DCs was demonstrated recently.35 Thus, MGL functions as an efficient antigen-endocytic receptor on DCs and is able to enhance DC functions through recognition of Tn antigen. Indeed, CLRs have been widely studied as DC targets and constitute promising new targets for enhancing vaccine effectiveness.36 The MAG:Tn vaccine is original in its use of a targeted glycosidic antigen for immunotherapy. It behaves as a targeting tool/vector for promoting immune responses and likely contributes to its efficacy for antitumor immunity.20,22 The use of antigen intrinsically able to target DCs is of particular interest for the design of anticancer vaccines.
When administered intradermally, glycopeptides bearing the Tn antigen were captured in a selective manner by mouse CD103− skin DCs. Then, targeted MGL+ skin DCs delivered antigen to DLN where dermal DCs favored a Th2 response and the expansion of GC B cells for anti-Tn–specific antibody production. The immune responses elicited by targeting different DC surface molecules may differ for several reasons. The DC subset targeted may be specialized in particular functions, according to the intracellular compartment to which the antigen is delivered, leading to different presentation pathways. For instance, CD8α+ DCs are believed to play a dominant role in cross-presentation.10 Thus, antigen targeting to DEC205 on CD8α+ DCs and DCIR2 on CD8α− DCs would favor antigen MHC class I cross-presentation to CD8+ T cells or MHC class II presentation to CD4+ T cells, respectively.10 After herpes simplex virus infection, only CD8+ lymph node DCs and CD103+ dermal DCs are capable of efficient MHC class I cross-presentation and cytotoxic T lymphocyte (CTL) induction, whereas all DC subsets efficiently present antigen via MHC class II molecules.37 Similarly, in the respiratory tract, CD103+ DCs cross-present antigen to CD8+ T cells, whereas both CD103+ and CD11bhi CD103− DCs present viral antigen to CD4+ T cells.38 It should, however, be mentioned that CD103− CD11bhi DCs were recently reported as critical in CD8+ T-cell population expansion after pulmonary influenza infection.39
It has been also suggested that dermal skin DCs would be involved in B-cell priming because they migrate to the outer paracortex of the T-cell zone in the DLN, just beneath B-cell follicles.40 This migration might explain high levels of GC B cells induced by Tn glycopeptides. It seems likely that MGL+ CD103− dermal DCs are specialized in the induction of Th2 responses and favor an antibody-based immune response. This observation is consistent with recently published data describing specialized functions for different skin DC subsets.41 In human skin, 2 dermal DC subsets, CD1a+ DCs and CD14+ DCs, have been described, in addition to LCs. Dermal-like CD14+ DCs and CD14+ DCs from dermis were both shown to promote the generation of follicular helper T cells able to induce naive B cells to switch isotypes and produce large amounts of antibodies, constituting a skin DC subset specialized in the control of mature B-cell differentiation.42 Whether MGL expression found on dermal-like CD14+ DCs is also true for dermal CD14+ DCs is yet to be determined. Histologic studies suggest that MGL would be preferentially associated with CD1a+ DCs,43 a subset with a much less clear function.42
Antigen presentation may also depend on how DCs acquire antigen and the type of antigen they encounter. It may also depend on innate signals triggered by CLRs, which can alter T-cell polarization. Various studies have shown that antigen targeting to different molecules results in the induction of qualitatively different immune responses. For instance, Dectin-1 targeting favors the induction of IFN-γ by CD4+ and CD8 T+-cell subsets.44 To our knowledge, however, no innate signaling pathway has been attributed to MGL on DCs.
The specific recognition of glycosylated Tn antigen by MGL on DCs and the ability of these cells to present glycosylated Tn molecules raises questions about the role of MGL+ DCs in the recognition of cancer cells expressing this tumor-associated antigen. Indeed, recombinant MGL was shown to bind strongly to adenocarcinoma and melanoma cell lines.45 Moreover, MGL+ cells have been detected at tumor sites expressing Tn,46 suggesting that MGL+ cells may interact with tumor cells. Our results, therefore, suggest that MGL may act as a sensor for cells with aberrant glycosylation of surface molecules. Indeed, aberrant glycosylation is a hallmark of malignant cells.47 The Tn antigen is also strongly expressed by some parasites45,48 and may participate in parasite-specific immune responses.
In conclusion, the receptor MGL functions as an efficient endocytic-antigen receptor on DCs, taking up Tn-glycopeptides and glycoproteins and delivering them for processing and MHC class II antigen presentation to T cells, leading to the induction of CD4+ Th2 cells and Tn-specific B-cell responses. Thus, in vivo DC-targeting strategies based on Tn-MGL interactions represent a double-edged strategy, in which the promiscuous Tn tumor antigen is capable of efficiently targeting dermal DCs. Moreover, the density of the Tn ligand on MAG-Tn glycopeptides and its dendrimeric structure, could positively affect the efficacy of its capture and endocytosis by MGL+ cells and also induce the cross-linking of the MGL receptor. DC-targeting strategies based on Tn-MGL interactions would therefore constitute a promising approach for enhancing antigen presentation and Tn-specific immunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Laleh Majlessi and Gilles Dadaglio for critical reading of the manuscript.
This work was supported in part by grants from the Institut Pasteur (PIC-PTR 278), the Ligue Nationale Contre le Cancer (Equipe Labellisée 2007), Institut National du Cancer (Cancéropôle Ile-de-France) and MGEN Union to C.L. This work was also supported by the Fondation pour la Recherche Médicale and by Institut Pasteur to T.F. Finally, this work was also supported by Institut Pasteur (PTR 260) and Agence Nationale pour la Recherche (ANR R09111JS (Neosyk) to X.Z.
Authorship
Contribution: T.F. and X.Z. contributed equally to project design, research, and writing the paper; R.L.-M., S.B., and C.L. designed research and wrote the paper, with R.L. and S.B. contributing equally; E.D., C.G., and S.V.-G. also performed research; and E.A. and O.L. helped with experiment design for human dendritic cells.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claude Leclerc, Unité de Régulation Immunitaire et Vaccinologie, Institut Pasteur, 25-28 Rue du Dr Roux, 75724 Paris Cedex 15, France; e-mail: claude.leclerc@pasteur.fr.
References
Author notes
T.F. and X.Z. contributed equally to this work.

![Figure 3. Tn-glycosylated antigens target dermal dendritic cells (dDCs) in vivo. (A) Analysis of macrophage galactose-type lectin expression (bold line) in Langerhans cells (CD11c+ major histocompatibility complex [MHC]) II+ epithelial-cell adhesion molecule positive [EpCam+] CD11b+ Langerin+ CD103−), CD103+ dDC (CD11c+ MHCII+ EpCam− CD11b−), and CD103− dDC (CD11c+ MHCII+ EpCam− CD11b+ CD103−) from BALB/c mouse skin. Isotype control staining is shown as a gray histogram. (B) CD45+ CD11c+ cells from the dermis were incubated at 4°C or 37°C with Alexa647-labeled antigen glycosylated multiple antigenic glycopeptide (MAG):Tn3-poliovirus (PV; left panel), MUC6:Tn(MCF7) (right panel), or nonglycosylated controls MAP-PV and MUC6, respectively. Antigen binding and uptake were analyzed in the presence or absence of ethylenediaminetetraacetic acid, anti–macrophage galactose-type lectin monoclonal antibody (ERMP23), or control Ig2a (FACS-Aria II; BD Biosciences). (C) Interstitial DCs from dermis were incubated with glycosylated MAG:Tn3-PV or nonglycosylated multiple antigenic peptides-PV and cultured with a PV-specific T-cell hybridoma. IL-2 release by the T-cell hybridoma was analyzed in the 24-hour supernatant by enzyme-linked immunosorbent assay. (D) Alexa647- or (E) Alexa488-labeled MAG:Tn glycopeptides or nonglycosylated multiple antigenic peptides-PV were injected intradermally into the right ear of mice. (D) Eighteen hours later, antigen capture was analyzed by flow cytometry in CD45+ CD11c+ Langerin− cells present in the dermis, by comparison with antigen-free dermis of the left ear. (E) Alternatively, cells that have captured labeled antigen were directly analyzed from skin by gating on Langerhans cells (gray line), CD103+ (dashed line), and CD103− (bold line) dDCs. Mean fluorescence intensities (MFI) are shown in the boxes. Representative results from 3 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/18/10.1182_blood-2010-04-279133/4/m_zh89991060830003.jpeg?Expires=1769215265&Signature=F8FAsxXVm5vZNBz6rkjgN0REuBhT56zGYUDndQS5-QNFQ7F2hAB4~BOKxuYLaGG81EK9tWOXcxBVJvMM1Z~AEjcenadUDAyXPceuuBgtbsTBjMfT-B-gd0NpQ3qrXUIOpFWeOt4ouGnj9-phT04U2PNcgK2sxlUvaIpT2RhQ0gvYlKtVRSBbw3qT8SALr-1nSGzwjSyMux3vpFyr4fiDbpSCD5EfBTASwyxQTwAaf2qz9Bm5npoaWjO4jGps1k82kQWGCHKfRf7z4mR1ZyVXnKLRoc-Zng09KFpTYMiQNZEO78rzd-sdACH8E5Gw1ZhggHFf0c9NBgxSTsF5Qbl4wQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. CD103− dermal dendritic cells (dDCs) capture and transport glycosylated Tn antigen to the draining lymph nodes (DLN) for presentation to T cells. (A) Analysis of macrophage galactose-type lectin expression (bold line) in skin DLN DCs: CD8α+ DCs (CD11c+ major histocompatibility complex [MHC]) II+ CD11b− CD8α+), Langerhans cells (CD11c+ MHCII+ CD8α− Langerin+ CD103−), CD103+ dDC (CD11c+ MHCII+ CD8α− Langerin+ CD103+), and CD103− dDC (CD11c+ MHCII+ CD11b+ Langerin− CD103−). Isotype control staining is shown as a gray histogram. (B-C) PBS, 10 nmol Alexa647-labeled glycosylated Tn antigen, or nonglycosylated control antigen was injected intradermally into the mouse ear. After 24 hours, we analyzed skin DLN, non-DLN, or spleen total CD11c+ DCs (B) and DLN DC subsets migrating from the skin (C) for antigen capture by flow cytometry. (D-E) We injected mice intradermally, in both ears, with 10 nmol multiple antigenic glycopeptide:Tn3-poliovirus (PV), multiple antigenic glycopeptide:Tn6-PV, nonglycosylated multiple antigenic peptides-PV peptide, or PBS. Total CD11c+ DCs (D) and DLN DC subsets migrating from the skin (E) were purified and cultured with a PV-specific T-cell hybridoma 24 hours later. IL-2 release by the T-cell hybridoma was analyzed in the 24-hour supernatants by enzyme-linked immunosorbent assay. Data are expressed as mean ± SD of duplicates. Representative results from 2 experiments are shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/18/10.1182_blood-2010-04-279133/4/m_zh89991060830004.jpeg?Expires=1769215265&Signature=l4rxv4cPlxixt8Cyq-jm0PYZ4C5~olNffWogVa0nm66dZtOgiAwErf4jsnsU1kMHY5jRpyKdfaIs7mr0o5eTzon3Wm7eOy93TycNQUSIEOgsdst4BuNIJiJuAmqAqtNBm7rN6yJK8ylXqJ~x5g7aST-LUjwGzp2c3oE1IqurPRRWEmEf1CaY6kOeRwujfhx2zao90kIel6tNFJhwVfMSO5U0qSqCy3bwBa1aDAtJGzHc6FWBD~buHIYZe8jBypqGRja1YErwd54MubBATEexihz4hkcIHT5~44mZd2CfOEwCZ0OOfvh~~P1ikINpkXpBBRqrhNfcCe69Gz5t7RmmQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal