Abstract
In light-chain (AL) amyloidosis, prognosis is dictated by cardiac dysfunction. N-terminal natriuretic peptide type B (NT-proBNP) and cardiac troponins (cTn) are used to assess the severity of cardiac damage. We evaluated the prognostic relevance of a high-sensitivity (hs) cTnT assay, NT-proBNP, and cardiac troponin I in 171 consecutive patients with AL amyloidosis at presentation and 6 months after treatment. Response and progression of NT-proBNP were defined as more than 30% and more than 300 ng/L changes. All 3 markers predicted survival, but the best multivariable model included hs-cTnT. The hs-cTnT prognostic cutoff was 77 ng/L (median survival 10.6 months for patients with hs-cTnT above the cutoff). After treatment, response and progression of NT-proBNP and a more than 75% increase of hs-cTnT were independent prognostic determinant. In AL amyloidosis, hs-cTnT is the best baseline prognostic marker. Therapy should be aimed at preventing progression of cardiac biomarkers, whereas NT-proBNP response confers an additional survival benefit.
Introduction
In light-chain (AL) amyloidosis, a hematologic malignancy causes multiorgan dysfunction, posing unique challenges to the hematologist.1 Organ damage increases the susceptibility to treatment toxicity, whereas outcome depends on the ability to rapidly restore organ function. Thus, there is the need of accurate markers for risk stratification and organ response, to guide the choice of therapy and assess its efficacy.2 The most important prognostic determinant in AL amyloidosis is heart dysfunction, and its severity is best assessed by the cardiac biomarkers N-terminal natriuretic peptide type B (NT-proBNP) and cardiac troponins (cTn).3,4 The combination of these biomarkers allows prognostic stratification.5 Moreover, the reduction of NT-proBNP is associated with hematologic response and prolonged survival.6
Recently, a new generation of high-sensitivity (hs) assays for cTn has been developed to identify minimal cardiac damage in the setting of acute coronary syndromes.7-9 Even slight elevations of cTn are associated with poor outcome in ischemic heart disease, indicating a role of hs-cTn also in prognostic stratification.8 Moreover, hs-cTn assays can detect circulating cTn also in patients with heart failure and nonischemic heart disease.10
It has been recently proposed that studies of novel markers of cardiovascular risk should report the degree to which they add to the information already provided by standard markers, emphasizing that no single statistical measure provides all the information.11 In the present study, we investigated the added prognostic value of high-sensitivity cardiac troponin T (hs-cTnT) in patients with AL amyloidosis compared with established risk markers according to these new criteria and its utility in determining the cardiac response to therapy.
Methods
Patients
A total of 171 consecutive newly diagnosed patients with AL amyloidosis enrolled between January 2004 and May 2008 were retrospectively evaluated. All patients gave written informed consent according to the Institutional Review Board guidelines and the Declaration of Helsinki. The Institutional Review Board of the Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo approved the storage and use of biologic samples and the use of patients' clinical data for research purposes. Organ involvement and response to therapy were defined according to the 2005 consensus criteria.12 In particular, heart involvement was defined as mean left ventricular wall (mLVW) thickness more than 12 mm at echocardiography in the absence of other potential causes of left ventricular hypertrophy, and hematologic response as a more than 50% decrease of involved (amyloidogenic) circulating free light chains (iFLCs). In patients in whom baseline iFLCs were less than 100 mg/L and who had a measurable more than 5 g/L serum monoclonal component, hematologic response was defined as a more than 50% reduction of the monoclonal component. Based on the biologic variability of NT-proBNP13,14 and on previous studies in AL amyloidosis,6,15 response and progression of NT-proBNP were defined as changes both more than 30% and more than 300 ng/L. A baseline NT-proBNP more than or equal to 650 ng/L was required for NT-proBNP response to be evaluable. Response to therapy was assessed 6 months after treatment initiation. Patients were treated with oral melphalan plus dexamethasone (109, 64%),16 cyclophosphamide, thalidomide, and dexamethasone (22, 13%),17 dexamethasone alone (17, 10%),18 autologous stem cell transplantation (18, 10%), and other treatments (5, 3%). Four patients were not evaluable for response because they died before 6 months.
Analytical methods
The hs-cTnT was measured on the Modular E instrument with a precommercial immunoassay from Roche Diagnostics on frozen sera. The 99th centile of hs-cTnT concentration in sera from 546 healthy volunteers is 14 ng/L (95% confidence interval, 12.4-24.9 ng/L). The analytical performance of the assay has been recently validated.19 Serum NT-proBNP and cardiac troponin I (cTnI) were measured on fresh sera with an electrochemiluminescence sandwich immunoassay (Roche Diagnostics) and a chemiluminescence assay (Advia Centaur CP, Siemens Diagnostics), respectively. The upper reference limits of NT-proBNP in men and women are, respectively, 88 ng/L and 153 ng/L in subjects younger than 50 years and 227 ng/L and 334 ng/L in persons 50 years of age and older. A serum cTnI more than 40 ng/mL indicates minimal myocardial lesion (99th centile of the reference population as reported by the manufacturer).
Statistical analysis
Continuous data were described with median and range. Biomarkers were dichotomized according to their upper reference limits, the prognostic cutoffs previously reported for AL amyloidosis,5 or using receiver operator characteristic (ROC) curve analysis to identify the best cutoffs for death at 12 months. For NT-proBNP, the reference limits vary with sex and age and are very close to the prognostic cutoff proposed by Dispenzieri et al (332 ng/L).5 Thus, we used the latter for the purpose of survival analysis. The patients were stratified according to the Mayo Clinic staging system: stage I patients had both NT-proBNP less than 332 ng/L and cTnI less than 100 ng/L, stage II patients had either NT-proBNP more than or equal to 332 ng/L or cTnI more than or equal to 100 ng/L, and stage III patients had both NT-proBNP more than or equal to 332 ng/L and cTnI more than or equal to 100 ng/L.5 Cox models were fitted to compute hazard ratios and 95% confidence intervals for death for a series of potential predictors. For evaluating the role of responses at 6 months, time-dependent Cox models were fitted. The proportional hazard assumption was tested and satisfied in all cases. To measure model performance, the Harrell concordance (c) statistic (for discrimination between cases with and without the outcome, ie, death, equivalent to the area under the ROC curve), the shrinkage coefficient for calibration (measuring the accuracy of the model, ie, the agreement between observed outcome and prediction), and the Royston explained variation (R2, ability to account for the variation in the outcome observed in the population) were computed (for all, the higher, the better). Model fit was graphically assessed by Snell residuals. Multivariable models were used, including noncollinear predictors showing a P less than .01 at univariable analysis. The final model based on dichotomized, rather than continuous, predictors was preferred for its easier clinical use and better fit to the data. To account for the possible effect of renal failure on the concentration of the cardiac biomarkers, particularly NT-proBNP, and of iFLC, all the Cox analyses were repeated after stratification for renal function according to a 60 mL/min per 1.73 m2 estimated glomerular filtration rate (eGFR) cutoff. The ability of changes in the cardiac biomarkers to discriminate patients who died at 1 year after evaluation of response was assessed by ROC analyses. Survival curves were plotted according to Kaplan-Meier and differences in survival tested for statistical significance by the log-rank test. Stata 11 (StataCorp) and MedCalc 11 (Medcal Softwarem) were used for computation.
Results
A total of 171 consecutive untreated patients with AL amyloidosis were included in the study. The patients' characteristics are reported in Table 1. It is worth noting that no patient with heart involvement had normal NT-proBNP, in agreement with the reported 100% diagnostic sensitivity of this biomarker.3
Characteristics of 171 patients with AL amyloidosis
| Variable . | n (%) . | Median (range) . |
|---|---|---|
| Age, y | — | 64 (38-84) |
| Male sex | 100 (58) | — |
| Kidney involvement | 127 (74) | — |
| Heart involvement (mLVW thickness > 12 mm) | 119 (70) | — |
| Peripheral nervous system involvement | 40 (23) | — |
| Liver involvement | 30 (17) | — |
| > 1 organ involved | 141 (82) | — |
| iFLC > 125 mg/L | 89 (52) | — |
| NYHA class > 2 | 64 (37) | — |
| EF < 55% | 52 (30) | — |
| eGFR, mL/min per 1.73 m2 | — | 68 (9-136) |
| eGFR < 60 mL/min per 1.73 m2 | 62 (36) | — |
| hs-cTnT, ng/L | — | 31 (3-420) |
| hs-cTnT > 14 ng/L | 121 (71) | — |
| hs-cTnT > 77 ng/L | 39 (23) | — |
| cTnI, ng/L | — | 40 (0-85) |
| cTnI > 40 ng/L | 79 (46) | — |
| cTnI > 70 ng/L | 40 (23) | — |
| cTnI > 100 ng/L | 31 (18) | — |
| NT-proBNP, ng/L | — | 2591 (25-35 563) |
| NT-proBNP > 332 ng/L | 134 (78) | — |
| NT-proBNP > 2736 ng/L | 64 (37) | — |
| Mayo Clinic stage I (NT-proBNP < 332 ng/L and cTnI < 100 ng/L) | 37 (22) | — |
| Mayo Clinic stage II (NT-proBNP ≥ 332 ng/L or cTnI ≥ 100 ng/L) | 103 (60) | — |
| Mayo Clinic stage III (NT-proBNP ≥ 332 ng/L and cTnI ≥ 100 ng/L) | 31 (18) | — |
| Variable . | n (%) . | Median (range) . |
|---|---|---|
| Age, y | — | 64 (38-84) |
| Male sex | 100 (58) | — |
| Kidney involvement | 127 (74) | — |
| Heart involvement (mLVW thickness > 12 mm) | 119 (70) | — |
| Peripheral nervous system involvement | 40 (23) | — |
| Liver involvement | 30 (17) | — |
| > 1 organ involved | 141 (82) | — |
| iFLC > 125 mg/L | 89 (52) | — |
| NYHA class > 2 | 64 (37) | — |
| EF < 55% | 52 (30) | — |
| eGFR, mL/min per 1.73 m2 | — | 68 (9-136) |
| eGFR < 60 mL/min per 1.73 m2 | 62 (36) | — |
| hs-cTnT, ng/L | — | 31 (3-420) |
| hs-cTnT > 14 ng/L | 121 (71) | — |
| hs-cTnT > 77 ng/L | 39 (23) | — |
| cTnI, ng/L | — | 40 (0-85) |
| cTnI > 40 ng/L | 79 (46) | — |
| cTnI > 70 ng/L | 40 (23) | — |
| cTnI > 100 ng/L | 31 (18) | — |
| NT-proBNP, ng/L | — | 2591 (25-35 563) |
| NT-proBNP > 332 ng/L | 134 (78) | — |
| NT-proBNP > 2736 ng/L | 64 (37) | — |
| Mayo Clinic stage I (NT-proBNP < 332 ng/L and cTnI < 100 ng/L) | 37 (22) | — |
| Mayo Clinic stage II (NT-proBNP ≥ 332 ng/L or cTnI ≥ 100 ng/L) | 103 (60) | — |
| Mayo Clinic stage III (NT-proBNP ≥ 332 ng/L and cTnI ≥ 100 ng/L) | 31 (18) | — |
— indicates not applicable; NYHA, New York Heart Association; and EF, ejection fraction.
Prognostication of survival at presentation
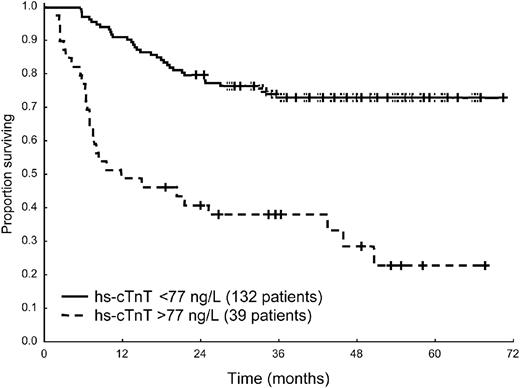
Fifty-six patients (33%) died. The median follow-up of living patients was 49 months (range, 19-70 months). The cutoffs best predicting death at 1 year were 77 ng/L for hs-cTnT (Figure 1), 70 ng/L for cTnI, 2736 ng/L for NT-proBNP, 125 mg/L for iFLC, 12 mm for mLVW, and 55% for ejection fraction. The Mayo Clinic staging system based on NT-proBNP and cTnI divided the patient population in 3 groups with significantly different survival (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The 39 patients with hs-cTnT more than 77 ng/L were distributed as follows: 1 of 37 subjects (3%) in stage I, 10 of 103 (10%) in stage II, and 28 of 31 (90%) in stage III. The survival of the 10 stage II patients with elevated hs-cTnT was not significantly different from that of stage III patients (supplemental Figure 1B). At Cox univariable analysis, all the 3 cardiac biomarkers significantly predicted mortality, as well as the echocardiographic variables and the concentration of iFLC (Table 2).
Survival from diagnosis of 171 patients according to hs-cTnT concentration at presentation. Median survival 10.6 months versus not reached, P < .001.
Survival from diagnosis of 171 patients according to hs-cTnT concentration at presentation. Median survival 10.6 months versus not reached, P < .001.
Cox analysis of survival
| Variable . | HR (95% CI) . | P . |
|---|---|---|
| Univariable analysis | ||
| Age | 1.02 (0.99-1.04) | .203 |
| Male sex | 1.39 (0.82-2.33) | .219 |
| ln(iFLC) | 1.22 (1.02-1.46) | .024 |
| iFLC > 125 mg/L | 2.00 (1.19-3.37) | .009 |
| NYHA class > 2 | 3.05 (1.83-5.06) | < .001 |
| mLVW thickness | 1.14 (1.06-1.22) | .001 |
| mLVW thickness > 12 mm | 2.84 (1.44-5.59) | .003 |
| EF | 0.96 (0.93-0.98) | .001 |
| EF < 55% | 1.62 (0.97-2.70) | .064 |
| ln(cTnI) | 1.59 (1.26-2.01) | < .001 |
| cTnI > 40 ng/L (url) | 2.85 (1.55-5.24) | .001 |
| cTnI > 70 ng/L | 3.90 (2.16-7.05) | < .001 |
| cTnI > 100 ng/L | 4.64 (2.51-8.58) | < .001 |
| ln(NT-proBNP) | 1.42 (1.20-1.68) | < .001 |
| NT-proBNP > 332 ng/L | 4.06 (1.63-10.14) | .003 |
| NT-proBNP > 2736 ng/L | 2.63 (1.60-4.34) | < .001 |
| ln(hs-cTnT) | 1.78 (1.40-2.27) | < .001 |
| hs-cTnT > 14 ng/L (url) | 2.71 (1.37-5.34) | .004 |
| hs-cTnT > 77 ng/L | 4.33 (2.61-7.18) | < .001 |
| Multivariable analysis of baseline variables | ||
| iFLC > 125 mg/L | 1.72 (1.01-2.91) | .045 |
| NYHA class > 2 | 2.20 (1.23-3.95) | .008 |
| mLVW thickness > 12 mm | 1.36 (0.63-2.92) | .428 |
| EF < 55% | 1.01 (0.59-1.74) | .967 |
| hs-cTnT > 77 ng/L | 3.83 (2.82-6.41) | < .001 |
| Time-dependent multivariable analysis, including baseline variables and hematologic and NT-proBNP response | ||
| iFLC > 125 mg/L | 1.34 (0.75-2.41) | .320 |
| NYHA class > 2 | 1.87 (1.02-3.43) | .043 |
| mLVW thickness > 12 mm | 1.60 (0.72-3.54) | .245 |
| hs-cTnT > 77 ng/L | 4.55 (2.54-8.16) | < .001 |
| NT-proBNP response | 0.45 (0.18-1.10) | .074 |
| NT-proBNP progression | 3.79 (1.84-8.00) | < .001 |
| hs-cTnT increase > 75% | 3.31 (1.81-6.04) | < .001 |
| Hematologic response | 0.69 (0.35-1.37) | .295 |
| Variable . | HR (95% CI) . | P . |
|---|---|---|
| Univariable analysis | ||
| Age | 1.02 (0.99-1.04) | .203 |
| Male sex | 1.39 (0.82-2.33) | .219 |
| ln(iFLC) | 1.22 (1.02-1.46) | .024 |
| iFLC > 125 mg/L | 2.00 (1.19-3.37) | .009 |
| NYHA class > 2 | 3.05 (1.83-5.06) | < .001 |
| mLVW thickness | 1.14 (1.06-1.22) | .001 |
| mLVW thickness > 12 mm | 2.84 (1.44-5.59) | .003 |
| EF | 0.96 (0.93-0.98) | .001 |
| EF < 55% | 1.62 (0.97-2.70) | .064 |
| ln(cTnI) | 1.59 (1.26-2.01) | < .001 |
| cTnI > 40 ng/L (url) | 2.85 (1.55-5.24) | .001 |
| cTnI > 70 ng/L | 3.90 (2.16-7.05) | < .001 |
| cTnI > 100 ng/L | 4.64 (2.51-8.58) | < .001 |
| ln(NT-proBNP) | 1.42 (1.20-1.68) | < .001 |
| NT-proBNP > 332 ng/L | 4.06 (1.63-10.14) | .003 |
| NT-proBNP > 2736 ng/L | 2.63 (1.60-4.34) | < .001 |
| ln(hs-cTnT) | 1.78 (1.40-2.27) | < .001 |
| hs-cTnT > 14 ng/L (url) | 2.71 (1.37-5.34) | .004 |
| hs-cTnT > 77 ng/L | 4.33 (2.61-7.18) | < .001 |
| Multivariable analysis of baseline variables | ||
| iFLC > 125 mg/L | 1.72 (1.01-2.91) | .045 |
| NYHA class > 2 | 2.20 (1.23-3.95) | .008 |
| mLVW thickness > 12 mm | 1.36 (0.63-2.92) | .428 |
| EF < 55% | 1.01 (0.59-1.74) | .967 |
| hs-cTnT > 77 ng/L | 3.83 (2.82-6.41) | < .001 |
| Time-dependent multivariable analysis, including baseline variables and hematologic and NT-proBNP response | ||
| iFLC > 125 mg/L | 1.34 (0.75-2.41) | .320 |
| NYHA class > 2 | 1.87 (1.02-3.43) | .043 |
| mLVW thickness > 12 mm | 1.60 (0.72-3.54) | .245 |
| hs-cTnT > 77 ng/L | 4.55 (2.54-8.16) | < .001 |
| NT-proBNP response | 0.45 (0.18-1.10) | .074 |
| NT-proBNP progression | 3.79 (1.84-8.00) | < .001 |
| hs-cTnT increase > 75% | 3.31 (1.81-6.04) | < .001 |
| Hematologic response | 0.69 (0.35-1.37) | .295 |
HR indicates hazard ratio; CI, confidence interval; NYHA, New York Heart Association; EF, ejection fraction; and url, upper reference limit.
Because the 3 cardiac biomarkers were highly correlated, they could not be included in a single multivariable model. Thus, we fitted 3 alternative models. The one including hs-cTnT (Table 2) turned out to be the model most accurately predicting the patients' outcome because it showed better agreement between observed and predicted deaths (shrinkage coefficient 0.91 for the model, including hs-cTnT, compared with 0.82 and 0.81 for those, including cTnI and NT-proBNP, respectively), and accounted for a greater proportion of the observed deaths (Royston explained variation 0.41 for the hs-cTnT model, compared with 0.33 for the cTnI and 0.24 the NT-proBNP ones). This indicates that hs-cTnT is a more powerful prognostic determinant than NT-proBNP and cTnI. Stratification for eGFR, to account for the effect of renal failure on the concentration of cardiac biomarkers, particularly NT-proBNP, and of iFLC, did not significantly change the results (not shown).
The performance of the univariable and multivariable models is reported in supplemental Table 1.
Prognostication of survival at the time of evaluation of response to chemotherapy
Hematologic response was achieved in 92 of 167 evaluable patients (55%), including 38 (23%) complete remissions. Among 113 patients with baseline NT-proBNP more than or equal to 650 ng/L, 43 (38%) achieved NT-proBNP response. Of the 43 patients who achieved NT-proBNP response, 23 (53%) were in complete remission, 18 (42%) were in partial hematologic response, and 2 (5%) were nonresponders. Progression of NT-proBNP was observed in 53 of 167 subjects (32%): 12 (23%) in partial hematologic response and 41 (77%) nonresponders. Only one patient, who obtained NT-proBNP response, achieved cardiac response as assessed by echocardiography (≥ 2 mm mLVW thickness reduction). A more than or equal to 2-mm increase in mLVW thickness was observed in 4 patients, all of whom also had NT-proBNP progression. A more than or equal to 20% decrease in ejection fraction was observed in no patient.
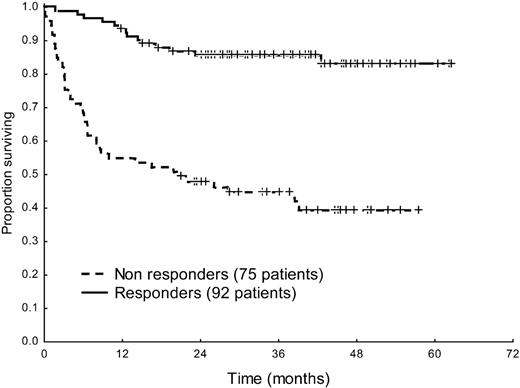
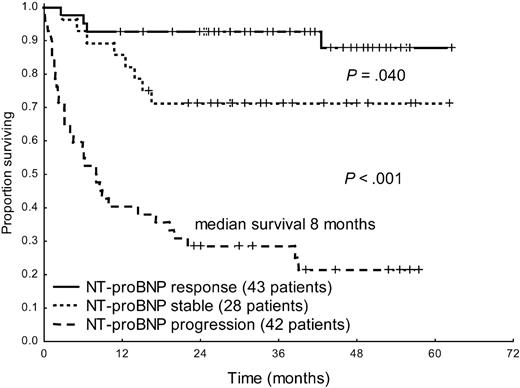
Hematologic response significantly improved survival in the overall patient population (median survival, 20 months vs not reached, P < .001, Figure 2). Moreover, response and progression of NT-proBNP could discriminate between 3 groups with significantly different survival (Figure 3). Interestingly, also patients with baseline hs-cTnT more than 77 ng/L significantly benefited from hematologic (median, 3 months vs not reached, P = .002) and NT-proBNP (median survival, 4 months vs not reached, P < .001) response. However, among the 53 patients in whom NT-proBNP progressed, partial hematologic response to treatment, which was observed in 12 cases (23%), did not induce a significant survival benefit (median, 8 vs 13 months, P = .34).
Survival from the time of evaluation of response of 167 patients according to hematologic response to treatment. Median survival 20 months versus not reached, P < .001.
Survival from the time of evaluation of response of 167 patients according to hematologic response to treatment. Median survival 20 months versus not reached, P < .001.
Survival from the time of evaluation of response of 113 patients with baseline NT-proBNP more than or equal to 650 ng/L according to NT-proBNP response and progression.
Survival from the time of evaluation of response of 113 patients with baseline NT-proBNP more than or equal to 650 ng/L according to NT-proBNP response and progression.
The ROC analyses based on death 1 year after evaluation of response showed that percentage and absolute changes in NT-proBNP better discriminated patients who died than changes in hs-cTnT, as assessed by comparison of the areas under the ROC curves (0.79 vs 0.61 for percentage variation, P = .004, and 0.83 vs 0.68 for absolute changes, P = .012). The hs-cTnT cutoff best predicting death after treatment was a more than 75% increase. Such a remarkable increase was observed only in 14 patients, 12 of whom (86%) also had an NT-proBNP progression. Eleven (79%) of the 14 patients in whom hs-cTnT increased by more than 75% died. Furthermore, a hs-cTnT increase more than 75% identified a subset of patients with a trend toward a shorter survival among those in whom NT-proBNP progressed (median, 8 vs 24 months, P = .063).
Hematologic response to therapy, NT-proBNP response and progression, and hs-cTnT progression, along with baseline variables were included in a time-dependent multivariable model stratified for renal function to take into account the effect of impaired renal function on the concentration of NT-proBNP and iFLC. In this model, the presence of heart failure at presentation, baseline hs-cTnT more than 77 ng/L, NT-proBNP progression, and hs-cTnT increase more than 75% were all independent prognostic determinants, and NT-proBNP response had borderline statistical significance (Table 2). The prognostic significance of NT-proBNP response was lower in the multivariable model without stratification for renal function (not shown).
Discussion
The role of baseline measurement of high-sensitivity cardiac troponin T
The present study showed that all 3 cardiac biomarkers tested are indicators of prognosis in AL amyloidosis. At univariable analysis, the cutoffs of NT-proBNP, cTnI, and hs-cTnT were significantly associated with survival, and it was possible to fit 3 multivariable models, each including one biomarker. This is in agreement with previous observations that NT-proBNP and cTnI are useful in prognosticating survival in AL amyloidosis. Nevertheless, statistical significance is not the only indicator of the performance of a marker of prognosis,11 and in our study, cardiac TnT measured with a high-sensitivity assay proved to be the best baseline prognostic marker in AL amyloidosis, showing higher accuracy in predicting the patients' outcome compared with cTnI and NT-proBNP. Using presenting hs-cTnT concentration as a single variable, it is possible to easily identify 2 groups of patients with strikingly different survival: 1 year after diagnosis 52% of patients with baseline hs-cTnT more than 77 ng/L die, compared with only 9% of other subjects. Moreover, the use of hs-cTnT may further refine the Mayo Clinic staging system. In our study, the survival of the 10 stage II patients with elevated hs-cTnT was not different from that of stage III patients, indicating that hs-cTnT may reclassify a proportion of Mayo Clinic stage II patients as high-risk subjects. However, given the low number of such patients in the present series, this observation needs confirmation from larger studies. Thus, hs-cTnT could be a simple, although powerful, tool both for single subjects risk assessment and for patient stratification in clinical trials. Nevertheless, response to treatment improves survival also in patients with initially elevated hs-cTnT, emphasizing the usefulness of treating also subjects with advanced cardiac damage using rapidly effective agents.
The role of measurement of cardiac biomarkers in the evaluation of response to treatment
Progression of cardiac biomarkers, defined as a more than 30% and more than 300 ng/L increase of NT-proBNP, and a more than 75% increase in hs-cTnT emerges as an important independent prognostic factor in AL amyloidosis. Hematologic response is of no benefit to patients in whom cardiac dysfunction (ie, NT-proBNP) progresses, indicating that the impact on survival of the reduction of the amyloidogenic light chain is dependent on its ability to induce cardiac response or at least to prevent cardiac progression. Thus, in AL amyloidosis, treatment efficacy should be monitored with cardiac biomarkers (NT-proBNP and hs-cTnT). The short-term aim of therapy should be to prevent an increase of NT-proBNP and/or hs-cTnT, whereas inducing a NT-proBNP response can confer additional benefit. In the presence of an elevation of NT-proBNP and/or hs-cTnT compared with baseline, the therapeutic decision should take into account the hematologic response: if the patient is refractory, a different therapeutic approach should be considered. Conversely, if a partial hematologic response has been attained, the possibility of continuing treatment to achieve a more pronounced reduction of amyloidogenic FLC with the aim of arresting cardiac progression should be considered. Ideally, the biomarkers should be monitored in parallel with the monitoring of the hematologic response. It has recently been observed that patients with AL amyloidosis treated with immunomodulatory drugs (IMiDs), such as thalidomide and lenalidomide, present an increase in NT-proBNP and cTnI that is probably treatment related. This increase in cardiac biomarkers was reported to be asymptomatic, and its mechanisms and prognostic impact have not been elucidated yet.20,21 However, early reports indicate that the effect of IMiDs appears as early as after the first cycle. Thus, frequent (eg, monthly) measurements of cardiac biomarkers may allow identification of a direct treatment effect underlying biomarker elevation. In the present series, 13% of patients were treated with thalidomide and none received lenalidomide. Excluding patients exposed to thalidomide did not change the results, but small numbers prevented us from performing a separate analysis in subjects who received this drug. Thus, caution should be used in interpreting biomarker progression in patients who are being treated with IMiDs. Validation studies in patient populations exposed to different types of treatments and, in particular, in subjects who receive IMiDs are warranted.
Additional caution should be used in the presence of other factors not related to amyloidosis or chemotherapy, for example, fluid overload, dehydration, or concomitant nonamyloid cardiac diseases, which may influence the concentration of cardiac biomarkers. The measurement of cardiac biomarkers, both at baseline and during the follow-up, should be done in a “steady state,” when optimal control of fluid retention has been achieved by supportive measures and in the absence of other concurrent conditions (such as the onset of atrial fibrillation) that may affect the concentration of cardiac biomarkers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Roche Diagnostics for providing the reagents for measurement of high-sensitivity troponin T.
This study was supported in part by the EURAMY project, which has received research funding from the Community's Sixth Framework Program; Fondazione Cariplo (Milano, Italy; Nobel Project, Transcriptomics and Proteomics Approaches to Diseases of High Sociomedical Impact: A Technology-Integrated Network), and Ricerca Finalizzata Malattie Rare, Ministero della Salute, Istituto Superiore di Sanità (526D/63), Ministry of Research and University (2007AESFX2_003) and Associzione Italiana per la Ricerca sul Cancro program “Harnessing tumor cell/microenvironment cross talk to treat mature B-cell tumors.”
Authorship
Contribution: G.P. and G.M. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript; A.B., R.P., R.A., R.M., and G.V.M.d. performed research, collected data, and contributed vital analytical tools; C.K. performed statistical analysis; and P.M., G.S., S.P., P.R., A.F., L.Z.B., and L.O. performed research and collected data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Giampaolo Merlini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo and University of Pavia, Viale Golgi, 19-27100 Pavia, Italy; e-mail: gmerlini@unipv.it.