Abstract
Fetal/neonatal alloimmune thrombocytopenia (FNAIT) resulting from fetal platelet destruction by maternal alloantibodies is the most common cause of severe fetal thrombocytopenia and of neonatal thrombocytopenia in maternity wards.1 The pathophysiology is largely unknown. The fetus has long been considered as an “innocent bystander.”
In this issue of Blood, Chen and colleagues, using murine models, demonstrate that the fetal, not maternal, major histocompatibility complex class I–related neonatal Fc receptor (FcRn) is implicated in the transplacental transfer of maternal antibodies and show that monoclonal antibody specific to FcRn may be effective in this disease.2
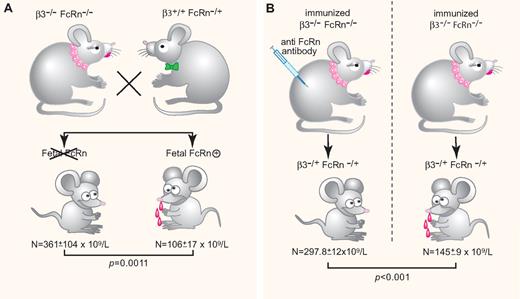
FcRn a key factor in FNIT. (A) Fetal FcRn is required for FNIT. Low platelet counts are observed only in pups expressing fetal FcRn delivered from immunized β3−/−FcRn−/− × β3+/+FcRn−/+. (B) Anti-FcRn monoclonal antibody injection during pregnancy. Low platelet counts are observed only in pups delivered from nontreated immunized female mice. N = platelet counts. (Professional illustration by Paulette Dennis.)
FcRn a key factor in FNIT. (A) Fetal FcRn is required for FNIT. Low platelet counts are observed only in pups expressing fetal FcRn delivered from immunized β3−/−FcRn−/− × β3+/+FcRn−/+. (B) Anti-FcRn monoclonal antibody injection during pregnancy. Low platelet counts are observed only in pups delivered from nontreated immunized female mice. N = platelet counts. (Professional illustration by Paulette Dennis.)
FNAIT (1/1000 live births) is usually discovered incidentally.3 The complication most feared is intracranial hemorrhage, leading to death or neurologic sequelae.4 If the fetus in a subsequent pregnancy is also platelet antigen incompatible, the condition is usually more severe, so antenatal management has been proposed with weekly maternal administration of intravenous immunoglobulins (IVIG).5 This treatment is relatively effective. However, this therapy relies on a human-derived product; it is expensive and in some cases therapy failures have been observed. Other approaches are therefore under study.6
Chen et al focus on the FcRn receptor, its role in the pathophysiology of fetal/neonatal immune thrombocytopenia (FNIT) and as a possible target for therapy. In fetal medicine, direct human research is usually not possible for ethical reasons. To address these questions the authors have developed several knockout murine models7 and monoclonal antibodies. The model they established is more similar to human platelet isoimmunization (the recipient lacks glyco-protein) than alloimmunization (immunization resulting from single amino-acid substitution); however, the major clinical effect of interest, that is, thrombocytopenia, is present in pups.
FcRn was characterized in the 1980s. This receptor is involved in the specific transport of IgG from the mother to the fetus. In rodents, FcRn is expressed on the cell-surface brush border of enterocytes. It has been shown that FcRn functions most efficiently in the neo-natal period when pups ingest maternal milk containing IgG.8 In humans, the maternal IgG transfer occurs antenatally via the placenta.
To identify more accurately the mechanisms responsible for mediating the transport of IgG maternal antibodies, Chen et al established a new model of FNIT using combined β3/FcRn-deficient mice. They document that anti-β3 integrin antibodies could be generated and maintained at a relatively high level in double β3/FcRn knockout mice immunized with β3 platelets. They demonstrate that FcRn is essential in the cross-placental passage of maternal antibodies by showing β3−/+FcRn+/+ pups having lower platelet counts than heterozygous β3 pups lacking FcRn. Chen and colleagues also reviewed the respective roles of maternal and fetal FcRn, clearly showing that fetal FcRn was the predominant effector for transplacental IgG transport and the resulting induction of low platelet counts in pups (see figure, panel A); in addition, they investigated whether FcRn may be a therapeutic target in FNIT.
Throughout life, FcRn plays a critical role in regulating the levels and persistence of IgG. Modulating the interaction of IgG with FcRn allows development of a new therapy in antibody-mediated diseases.9 To address this specific issue, Chen et al produced a new anti-FcRn monoclonal antibody. They found that pups delivered from anti-β3–immunized female mice treated with this antibody have higher platelet counts than those delivered from nontreated immunized mice (see figure, panel B), suggesting that such an antibody may be considered as a therapeutic agent.
The last part of the study examines the potential role of FcRn in the mechanisms involved in the action of IVIG.10 Reports favor saturation of FcRn, leading to increased catabolism of pathogenic antibodies.
Chen et al show that IVIG is able to down-regulate anti-β3 antibodies in immunized female mice—also in the absence of FcRn—and to increase platelet counts in pups delivered from IVIG-treated mice. This article makes new contributions, establishing that fetal FcRn is a key factor in the transplacental passage of maternal antibodies and may be considered as a potential therapeutic target. However, translating these results from murine models to humans should be done with caution; open questions remain. The placental structure varies remarkably across species. Humans and mice express different Fc receptors and FcRn-IgG interactions may differ between species. Animal models expressing human receptors and in vitro studies using human placental lobules are to be considered.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal