Abstract
African individuals harbor molecular RH variants, which permit alloantibody formation to high-prevalence Rh antigens after transfusions. Genotyping identifies such RH variants, which are often missed by serologic blood group typing. Comprehensive molecular blood group analysis using 3 genotyping platforms, nucleotide sequencing, and serologic evaluation was performed on a 7-year-old African male with sickle cell disease who developed an “e-like” antibody shortly after initiating monthly red blood cell (RBC) transfusions for silent stroke. Genotyping of the RH variant predicted a severe shortage of compatible RBCs for long-term transfusion support, which contributed to the decision for hematopoetic stem cell transplantation. RH genotyping confirmed the RH variant in the human leukocyte antigen–matched sibling donor. The patient's (C)ces type 1 haplotype occurs in up to 11% of African American sickle cell disease patients; however, haplotype-matched RBCs were serologically incompatible. This case documents that blood unit selection should be based on genotype rather than one matching haplotype.
Introduction
Red blood cell (RBC) transfusion is a common treatment for acute sickle cell disease (SCD)–related complications, and for both primary prevention of stroke and secondary stroke prophylaxis. RBC alloimmunization occurs in 18% to 47% SCD patients,1-3 compared with approximately 5% in thalassemia patients and 0.2% to 2.8% in the general population.2 Alloantibodies to C, E, and K antigens are most commonly involved, leading many transfusion centers to supply Rh and K phenotype-matched RBCs for SCD patients. Despite this practice, alloimmunization continues to complicate their RBC transfusions.
Reasons for the disproportionately high alloimmunization rates in SCD patients include in part disparate RBC antigens between donor and recipient due to clinically significant RH polymorphisms unrecognized by current serologic techniques.2 Individuals of African origin frequently harbor variants of RHD and RHCE genes. The absence of high-prevalence Rh antigens, like hrS (Rh19), hrB (Rh31), and HrB (RH34) in individuals homozygous for the variant predisposes them to Rh alloantibody formation after RBC transfusion,4,5 making the long-term transfusion support difficult to manage.
Hematopoetic stem cell transplantation (HSCT) has emerged as an alternative in many SCD patients who have severe disease, as a result of improved preparative regimens, graft sources, and reduction of HSCT-related side effects.6 Despite these advances, guidelines on the selection of and timing of SCD who would maximally benefit from HSCT have not been fully defined. Current consensus on HSCT indications include stroke, recurrent severe acute chest syndrome, chronic unremitting vaso-occlusive pain despite supportive care, or the inability to provide adequate supportive care such as chronic transfusion therapy or hydroxyurea.7
Molecular technology has advanced our knowledge of RH polymorphisms, and its application has made RBC genotyping a clinically useful tool, often with superior accuracy to serologic phenotyping.8 We present an informative patient history documenting the application of RBC genotyping in guiding both transfusion and HSCT donor selection strategy.
Case reports
A 7-year-old Cameroonian male with SCD (HbSS) and with magnetic resonance imaging (MRI) findings of silent stroke on routine screening was enrolled on the National Institutes of Health–funded Silent Cerebral Infarct Multi-Center Clinical Trial (Silent Infarct Transfusion, SIT study; ClinicalTrials.gov no. NCT00072761) and randomized to receive chronic transfusions. His RBC serologic phenotype was B Ccddee kk. After 8 leukocyte-reduced, Rh- and K-matched RBC transfusions from different donors, he developed a complicated alloantibody with “e-like” specificity. The antibody did not react like a simple alloanti-e (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). It did not represent an autoanti-e either because the patient's own RBCs were cross-match–compatible with the patient's antibody in the plasma. Cross-matching with D variants (supplemental Tables 2-3) and greater than 15 RBC units (not shown) indicated that compatible units would be difficult to obtain.
RBC genotyping confirmed an RH variant that is known to be associated with a severe shortage of compatible RBC supply. Because of the alloantibody formation, which rendered him incompatible with approximately 99.9% of RBC units, he was removed from the trial and transitioned to hydroxyrurea therapy, and the decision was made to proceed to a human leukocyte antigen (HLA)–matched related donor transplantation. HLA and RBC genotyping on 3 siblings revealed 2 siblings with full matches for 10 HLA antigens and who also possessed the RH variant of the patient. Both siblings were sickle cell trait and had stored cord allografts. After conditioning with alemtuzumab, fludarabine, and melphalan,9 the patient received a matched sibling donor combined umbilical cord and bone marrow transplant from his sister. His transplantation course was complicated by platelet refractoriness requiring HLA-matched platelets. His RBC antibody screen was negative at time of transplantation 13 months after the occurrence of the antibody, and he was supported with ccddee kk RBCs without evidence of hemolytic reactions. The patient remains well 1 year after transplantation without complications. He has a stable mixed hematopoietic chimerism with 89% donor leukocytes, a hemoglobin of 11.6 g/dL, quantitative hemoglobin S of 37%, and no antibodies to blood group antigens.
Methods
RBC genotyping analysis was performed using 3 commercially available genotyping platforms (Bloodchip, Progenika; Beadchip HEA version 1.2, Bioarray Solutions; and BAGene RH, Partial-D, Weak-D, D-zygosity, KKD, and MNS PCR-SSP kits, BAG) to assess the suitability of the available systems for the current clinical decision process (supplemental Table 4). Nucleotide sequencing using RHD- and RHCE-specific intronic primers was also performed (supplemental Tables 5-7).
Results and discussion
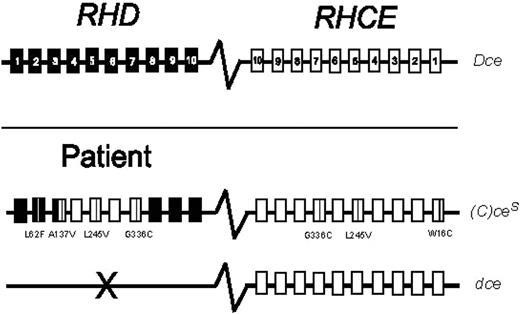
We performed RBC genotyping with 3 commercially available platforms (Table 1). The patient was found to be heterozygous for the (C)ces type 1 haplotype, with the common dce haplotype in trans. Nucleotide sequencing confirmed the (C)ces/dce genotype, and discriminated (C)ces type 1 from (C)ces type 2 by identifying a D/CE fusion point within RHD exon 3 along with 186 G>T (L62F), 410C>T (A137V), and 455A>C (N152T) nucleotide substitutions in exons 2 and 3 (Figure 1).
Rh phenotype prediction by genotyping with 3 molecular assays
| Antigens in the RH blood group system . | Genotyping platform . | ||
|---|---|---|---|
| Bloodchip . | Beadchip . | PCR-SSP . | |
| D | D− | NA | D−† |
| C/c | C+c+* | C−c+ | C−c+ |
| E/e | E−e+ | E−e+ | E−e+ |
| Cx | Cx− | NA | Cx− |
| Cw | Cw− | NA | Cw− |
| VS | VS+ | VS+ | VS+ |
| Antigens in the RH blood group system . | Genotyping platform . | ||
|---|---|---|---|
| Bloodchip . | Beadchip . | PCR-SSP . | |
| D | D− | NA | D−† |
| C/c | C+c+* | C−c+ | C−c+ |
| E/e | E−e+ | E−e+ | E−e+ |
| Cx | Cx− | NA | Cx− |
| Cw | Cw− | NA | Cw− |
| VS | VS+ | VS+ | VS+ |
NA indicates not available by platform
Although the results demonstrated a “cc” genotype, the “C+c+” phenotype is designated by this platform when associated with rs.
“D-zygosity” kit revealed 1 copy of normal Rhesus box, and one copy of hybrid Rhesus box consistent with cdes/d. RHD-CE(3–7)-D confirmed through use of “Partial-D” kit.
RH genotype of the patient with sickle cell disease. The common RH haplotype Dce is represented by the RHD and RHCE genes, which are homologous, oriented in opposite directions, and comprise 10 exons each (top panel). The patient's genotype harbors the variant (C)ces type 1 and the common dce haplotypes (bottom panel). The variant amino acid substitutions are shown by positions and amino acids involved, relative to the common RH alleles.
RH genotype of the patient with sickle cell disease. The common RH haplotype Dce is represented by the RHD and RHCE genes, which are homologous, oriented in opposite directions, and comprise 10 exons each (top panel). The patient's genotype harbors the variant (C)ces type 1 and the common dce haplotypes (bottom panel). The variant amino acid substitutions are shown by positions and amino acids involved, relative to the common RH alleles.
RBC genotyping contributed to the clinical decision to perform a hematopoietic stem cell transplantation (HSCT) in a 7-year-old Cameroonian boy with sickle cell disease (SCD). This patient's history points to a wider use for prospective RBC genotyping to guide medical decisions in SCD. Given that stable mixed chimerism can be expected in approximately 25% of SCD HSCTs with myeloablative conditioning,10 RBC genotyping may also be used to assist in HSC donor selection by identifying sibling donors who also possess the RH variant in order to prevent post transplantation chronic hemolysis or graft failure. Recently, RBC genotyping has been reported to impact HSCT decisions in a patient with chronic granulomatous disease (CGD), McLeod phenotype, and RBC alloantibodies.11 RBC genotyping has been implemented successfully in some institutions to facilitate the transfusion support of SCD patients with phenotypically matched RBCs12 ; however, its relevance as diagnostic guide for influencing the decisions regarding HSCT and donor selection is new.
The patient harbored the more prevalent variant of the 2 known (C)ces types, (C)ces type 1 (see supplemental materials for molecular details). Molecular screening of SCD patients in Europe and the southern US revealed (C)ces haplotype prevalence of 7.5% and 11.5%, respectively,4,13 which is much lower in a general donor population. Alloimmunization to high-prevalence Rh antigens in patients carrying (C)ces type 1 is common while such evidence is lacking in patients carrying (C)ces type 2. The risk and type of alloimmunization in individuals carrying a (C)ces haplotype is influenced by the second RH haplotype.5 Those with (C)ces/dce genotype may potentially produce alloanti-C.14
The “e-like” antibody within the plasma of the current patient was however unique. This complex alloantibody reacted selectively with high prevalence antigens of the Rh blood group, and was not an autoanti-e because the patient's own RBCs were cross-match–compatible with the patient's antibody in the plasma, the direct antiglobulin test (DAT) and elution of autologous RBCs after hypotonic washing as well as an auto-absorption were negative; among E antigen negative samples, only some RH variant RBCs were cross-match–compatible (supplemental Tables 1-3). The variable alloreactivity with many Rh variants precluded the continuation of chronic transfusion therapy because of the extreme difficulty to supply compatible RBCs, and demonstrates the need for further molecular characterization of the hrs and hrB antigens as well as donor screening.
A transfusion strategy using D negative (ccddee) RBC units was considered because the patient harbored the common dce haplotype, however the cross-match with ccddee RBC units was strongly positive. This case documents that knowledge of the genotype, which comprises both haplotypes, is useful.15
The current patient history demonstrates that prospective detection of RH variants, such as a (C)ces haplotype, through RBC genotyping offers several benefits to clinical decision making in SCD patients. Individuals at high risk of alloimmunization can be identified based on RH variant detection allowing clinicians to seek out alternatives to blood transfusion, like hydroxyurea or HSCT. More extensive blood donor screening by RBC genotyping for Rhesus and other blood group systems will allow the dry-matching of RBC units8 by identifying donors with compatible RH variants to provide the best compatible blood product. Identifying RH variants in HSCT donors may also be used for individuals undergoing HSCT, because prolonged stable mixed donor-host hematopoietic chimerism is expected to prevent chronic hemolysis in the posttransplantation setting.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mohamadou Sene for performing Beadchip HEA analysis.
The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the US Government.
Authorship
Contribution: R.M.F. participated in the patient care, performed sequencing experiments, analyzed and interpreted data, and wrote the manuscript; A.M. assisted in sequencing experiments and interpreted data; E.R.M. participated in the patient care, assisted in collecting samples and interpreting data; P.P. performed serologic evaluation and interpreted Beadchip HEA data; A.H.L.-S. performed and interpreted serologic evaluation and performed PCR-SSP genotyping; J.O. performed and interpreted Bloodchip experiments; H.G.K. interpreted data and contributed to writing; F.M.M. provided resources for sequencing experiments, interpreted data, and contributed to writing; N.R.K. participated in the patient care and contributed to writing; N.L.C.L. initiated the study, participated in the patient care, collected samples, and contributed to the interpretation of data and to writing; D.S. interpreted data and contributed to writing; and W.A.F. supervised PCR-SSP genotyping experiments, interpreted data, and cowrote the manuscript.
Conflict-of-interest disclosure: J.O. was an employee of Progenika Biopharma, which developed Bloodchip, one of the molecular blood group platforms used in the study. W.A.F. receives royalties and holds patents on RHD molecular genetics, participated in developing Bloodchip, and serves on the Scientific Advisory Board of Immucor. The remaining authors declare no competing financial interests.
The current affiliation for A.M. is Università di Roma “Sapienza” Dipartimento di Biotecnologie Cellulari ed Ematologia, Via Rovigo, Roma, Italy. The current affiliation for J.O. is BioFortis, Columbia, MD.
Correspondence: Willy A. Flegel, MD, Chief, Laboratory Services Section, Department of Transfusion Medicine, Clinical Center, National Institutes of Health, 10 Center Dr, Bethesda, MD 20892-1184; e-mail: flegelwa@cc.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal