Abstract
CXCR4 is a G protein–coupled chemokine receptor that has been implicated in the pathogenesis of primary immunodeficiency disorders and cancer. Autosomal dominant gain-of-function truncations of CXCR4 are associated with warts, hypo-gammaglobulinemia, infections, and myelokathexis (WHIM) syndrome, a primary immunodeficiency disorder characterized by neutropenia and recurrent infections. Recent progress has implicated CXCR4-SDF1 (stromal cell-derived factor 1) signaling in regulating neutrophil homeostasis, but the precise role of CXCR4-SDF1 interactions in regulating neutrophil motility in vivo is not known. Here, we use the optical transparency of zebrafish to visualize neutrophil trafficking in vivo in a zebrafish model of WHIM syndrome. We demonstrate that expression of WHIM mutations in zebrafish neutrophils induces neutrophil retention in hematopoietic tissue, impairing neutrophil motility and wound recruitment. The neutrophil retention signal induced by WHIM truncation mutations is SDF1 dependent, because depletion of SDF1 with the use of morpholino oligonucleotides restores neutrophil chemotaxis to wounds. Moreover, localized activation of a genetically encoded, photoactivatable Rac guanosine triphosphatase is sufficient to direct migration of neutrophils that express the WHIM mutation. The findings suggest that this transgenic zebrafish model of WHIM syndrome may provide a valuable tool to screen for agents that modify CXCR4-SDF1 retention signals.
Introduction
Stromal cell-derived factor 1 (SDF1, CXCL12)–mediated activation1 of the chemokine receptor CXCR4 is important for both normal and pathologic processes, including primordial germ cell migration, HIV pathogenesis, invasive migration of cancer cells, and leukocyte trafficking.2-5 Therefore, there is substantial interest in understanding how CXCR4-SDF1 signaling regulates cell motility and how these mechanisms can be targeted to treat human disease. CXCR4 signaling is attenuated by receptor internalization, which is regulated by phosphorylation events and binding of regulatory proteins to the cytoplasmic tail.6 The functional importance of CXCR4 internalization is highlighted by the dominantly inherited primary immunodeficiency, warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome, in which truncations of CXCR4 lead to altered signaling and gain of function.7-9
WHIM syndrome is characterized by warts, hypogammaglobulinemia, infections, and myelokathexis, a severe chronic neutropenia.10,11 Substantial evidence supports the importance of CXCR4 signaling in regulating neutrophil homeostasis and release from the bone marrow (BM).5 It has been postulated that the neutropenia in patients with WHIM syndrome results from both neutrophil retention in the BM and enhanced neutrophil apoptosis of retained neutrophils.11 Direct evidence to support this hypothesis has been provided by a mouse model of WHIM syndrome induced by the ectopic expression of WHIM truncation mutations of CXCR4 in hematopoietic stem cells that show impaired neutrophil release into the blood and increased rates of apoptosis in the BM.12 Previous reports indicate that neutrophils from patients with WHIM show increased signaling13 and chemotaxis8,9 in response to SDF1. However, some reports have suggested that the C-terminus of CXCR4 can both positively and negatively regulate cell motility8,9,14 and, alternatively, may be involved in modulating the precise targeting of cells in vivo.15
Despite the importance of CXCR4-SDF1 signaling, few animal models of WHIM syndrome are amenable to imaging or screening for drugs that modulate CXCR4-SDF1 function in vivo. Modeling WHIM syndrome is particularly attractive because CXCR4 signaling is important to many disease processes and is a direct result of aberrant chemokine signaling. Therefore, developing a model of WHIM syndrome in a system that allows the direct visualization of motility and chemotactic events in vivo would be a beneficial tool to understand disease pathogenesis. Our current understanding of WHIM syndrome is mostly derived from in vitro experiments8,9,13 or in vivo mouse models in which observing chemotactic events is difficult.12 The zebrafish, Danio rerio, is an ideal system to visualize neutrophil migration in vivo.16 In addition, there has been substantial recent progress in understanding how neutrophils develop17,18 and respond to inflammatory cues in zebrafish.16,19,20
Here, we describe the generation of a zebrafish model of the primary immunodeficiency syndrome, WHIM syndrome. WHIM zebrafish are neutropenic, and WHIM neutrophils show impaired recruitment to wounds and tissue inflammation, recapitulating the human disease. Exploiting the optical clarity of zebrafish larvae, we use real-time imaging to directly observe neutrophil motility in vivo. Expression of WHIM truncation mutations of CXCR4 in zebrafish neutrophils induces neutrophil retention in hematopoietic tissue and mediates a retention signal that is SDF1 dependent. These findings support the power of zebrafish to model primary immune disorders and provide a valuable tool to screen for agents that modify CXCR4-SDF1 retention signals.
Methods
Transient expression in zebrafish and generation of CXCR4b– GFP and WHIM–green fluorescent protein transgenic lines
All protocols that used zebrafish in this study were approved by the University of Wisconsin-Madison Research Animal Resources Center. All DNA expression vectors contain either the zebrafish myeloperoxidase (MPO) promoter for neutrophil expression16 or a cytomegalovirus promoter, minimal Tol2 elements for efficient integration,21 and an SV40 polyadenylation sequence (Clontech Laboratories Inc). Constructs with each of the following in the backbone were constructed: zCXCR4b–green fluorescent protein (GFP), zCXCR4b-WHIM-GFP, mCherry, SDF1a-2A-mCherry, and Dendra2 (Evergen). Expression of constructs was obtained by injection of 0.5-1 nL of 25 ng/μL plasmid with 45 ng/μL in vitro transcribed (Invitrogen) Tol2 transposase mRNA into the yolk of 1-cell–staged embryos just below the cell. Injected embryos were raised to sexual maturity and screened by crossing with wild-type fish to identify founders. F1 embryos were screened at 2-3 days postfertilization (dpf) for GFP expression in the caudal hematopoietic tissue (CHT) and raised to sexual maturity. Experiments were done on progeny of F1 incrosses. Transgenic lines from ≥ 2 separate founders were generated for each injected construct. All results reported here are from experiments performed on one of the transgenic lines, but many experiments were replicated on other lines to ensure consistency between founders. All experiments were done ≥ 3 times, and images of fixed larvae are representative of ≥ 20 larvae.
Tailfin wounding, whole-mount immunolabeling, and Sudan Black staining
For wounding assays larvae were anesthetized in E3 containing 0.1 mg/mL tricaine and wounded in the ventral tailfin with a 25-gauge needle, or their tails were transected with a surgical blade. After wounding, live larvae were embedded in 1% low-melt agarose for imaging or fixed for further processing. Larvae were fixed in 1% formaldehyde in phosphate-buffered saline overnight at 4°C and double-immunolabeled for MPO and L-plastin as described22 or fixed in 4% formaldehyde overnight at 4°C and stained with Sudan Black as reported.18
WISH
Plasmids containing mpo, sdf1a, and sdf1b were linearized with restriction enzymes (SalI for mpo and NotI for sdf1a and sdf1b) to serve as template for transcription of antisense probes. Digoxigenin-labeled RNA probes were transcribed for sdf1a and sdf1b with the use of T3 RNA polymerase. For double whole-mount in situ hybridization (WISH), fluorescein-labeled antisense mpo RNA probe was generated with T7 RNA polymerase. WISH was performed as described.23 After staining, embryos were placed in 80% glycerol for imaging.
MO microinjection
Morpholino oligonucleotides (MOs; Gene Tools) were resuspended in 1× Danieau buffer at a stock concentration of 1mM. Final MO concentrations were injected (0.5-1 nL) into the yolk of 1- to 2-cell–staged embryos.
Statistics
Experimental results were analyzed with the Prism version 4 (GraphPad Software) statistical software package. Statistical significance was determined with the unpaired Student t test (to compare 2 groups) or 1-way analysis of variance followed by Tukey multiple comparison post test (to compare multiple groups) at a 95% confidence interval. The resulting P values are included in the figure legends for each experiment; n = the number of larvae or neutrophils quantified.
Results
Hematopoietic expression of CXCR4b and SDF1a in zebrafish larvae
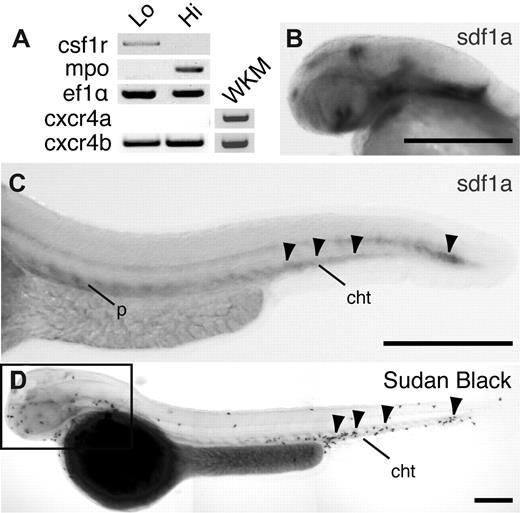
The zebrafish genome encodes 2 SDF1 ligands, SDF1a and SDF1b, and 2 SDF1 receptors, CXCR4a and CXCR4b. The expression pattern of CXCR4 in zebrafish hematopoietic cells has not previously been defined. To determine whether CXCR4 is expressed in zebrafish neutrophils, we performed reverse transcription–polymerase chain reaction for both CXCR4a and CXCR4b on RNA isolated from neutrophils and macrophages sorted from MPO:Dendra2 transgenic larvae at 3 dpf by fluorescence-activated cell sorting (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).22,24,25 As previously reported, cells with low levels of fluorescence represent macrophage-like cells and express the receptor CSF1R. In contrast, cells with high levels of fluorescence represent neutrophil-like cells and express MPO (Figure 1A).22 We found that both macrophages and neutrophils express CXCR4b but not CXCR4a. In contrast, both CXCR4a and CXCR4b are expressed in whole kidney marrow isolated from adult zebrafish (Figure 1A). These findings suggest that the primary SDF1 receptor in neutrophils from zebrafish larvae is CXCR4b.
Expression of CXCR4b and SDF1a in zebrafish larvae. (A) Reverse transcription–polymerase chain reaction of csf1r (macrophage marker), mpo (neutrophil marker), eflα (loading control), CXCR4a, and CXCR4b from MPO:Dendra2 high (hi) and low (lo) populations. WKM indicates whole kidney marrow from adult wild-type fish. (B-C) Whole-mount in situ hybridization of SDF1a expression in 2 dpf larvae, lateral view. Note SDF1a expression in the head (B) and CHT (C arrowheads). P indicates pronephric duct; cht, caudal hematopoietic tissue. (D) Whole-mount Sudan Black staining to visualize neutrophils in 2 dpf larvae, lateral view. Note neutrophil accumulation in areas of SDF1a expression in the head (box) and CHT (arrowheads). Bar = 200 μm (B-D).
Expression of CXCR4b and SDF1a in zebrafish larvae. (A) Reverse transcription–polymerase chain reaction of csf1r (macrophage marker), mpo (neutrophil marker), eflα (loading control), CXCR4a, and CXCR4b from MPO:Dendra2 high (hi) and low (lo) populations. WKM indicates whole kidney marrow from adult wild-type fish. (B-C) Whole-mount in situ hybridization of SDF1a expression in 2 dpf larvae, lateral view. Note SDF1a expression in the head (B) and CHT (C arrowheads). P indicates pronephric duct; cht, caudal hematopoietic tissue. (D) Whole-mount Sudan Black staining to visualize neutrophils in 2 dpf larvae, lateral view. Note neutrophil accumulation in areas of SDF1a expression in the head (box) and CHT (arrowheads). Bar = 200 μm (B-D).
To determine the spatial distribution of the CXCR4 ligand, SDF1, in zebrafish larvae, we performed WISH for SDF1a or SDF1b mRNA at 2 and 3 dpf. Interestingly, we observed concentration of SDF1a mRNA in regions of neutrophil production, including in the head (Figure 1B), the CHT (region of larval neutrophil development located in the anterior ventral tailfin), and along the pronephric duct (Figure 1C; supplemental Figure 1F-G), as previously reported.26 These areas of high SDF1a expression correlated with 2 distinct populations of neutrophils observed in zebrafish during this stage of development (Figure 1D). SDF1b was also expressed in the head and ventral body at 2 dpf, but staining was generally more diffuse (supplemental Figure 1B-C). Taken together, these findings suggest that zebrafish neutrophils develop or accumulate in regions with high SDF1a expression in zebrafish larvae.
Generation of WHIM-GFP transgenic zebrafish line
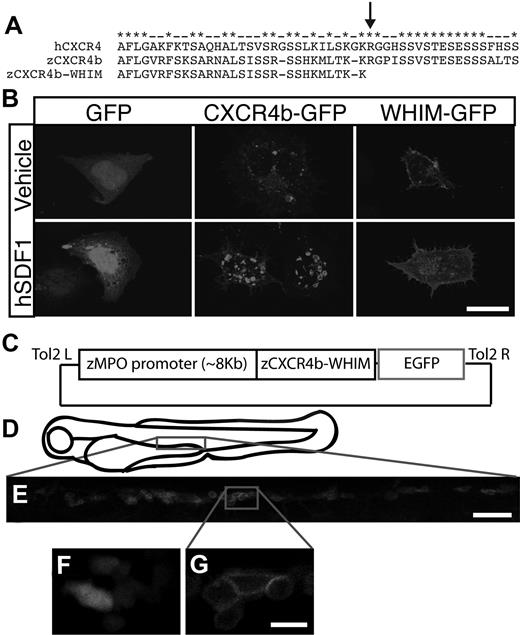
To model the human disorder, WHIM syndrome, in zebrafish we expressed the homologous CXCR4 receptor truncation mutations in zebrafish neutrophils with the use of the MPO promoter. Alignment of the C-terminal tail of zebrafish CXCR4b with human CXCR4 showed a high level of conservation, especially in serine residues that are critical for the proper internalization of CXCR4 after binding to SDF1 (Figure 2A).27 By truncating 19 amino acids from the C-terminus of CXCR4b, we generated a truncation mutation analogous to mutations described in patients with WHIM syndrome7 (Figure 2A). To determine whether this mutation affects receptor internalization we expressed both wild-type CXCR4b-GFP and the truncation mutant (hereafter referred to as WHIM-GFP) in human embryonic kidney cells and treated the cells with human SDF1. As expected both CXCR4b-GFP and WHIM-GFP localized to the cell membrane of transfected human embryonic kidney cells (supplemental Figure 2A). Treatment with SDF1 induced internalization of CXCR4b-GFP into intracellular vesicles, but not GFP or WHIM-GFP, indicating that the WHIM truncation impairs CXCR4b internalization after ligand binding (Figure 2B).
Generation of WHIM-GFP transgenic larvae. (A) Alignment of the C-terminal tails of human CXCR4 with zebrafish CXCR4b and WHIM-truncated zebrafish CXCR4b. Note conservation of serine residues. Arrow marks an identified WHIM truncation mutation. (B) Fluorescence images of human embryonic kidney cells expressing GFP (first column), CXCR4b-GFP (second column), or WHIM-GFP (third column) after incubation with human SDF1 (bottom row) or vehicle control (top row). (C) Schematic of Tol2-MPO:zCXCR4b-WHIM-GFP vector injected to generate WHIM-GFP transgenic lines. (D) Schematic of 3-dpf zebrafish larvae. Boxed region is approximate area magnified in panel E. (E) Fluorescence image of the CHT region of a 3-dpf WHIM-GFP larvae. (F-G) High-magnification image of GFP-expressing neutrophils from the CHT of a MPO:GFP (F) and a WHIM-GFP (G, blow up of box in larva from panel E. Note membrane expression in panel G. Bar = 50 μm (E); 20 μm (B); 10 μm (F-G).
Generation of WHIM-GFP transgenic larvae. (A) Alignment of the C-terminal tails of human CXCR4 with zebrafish CXCR4b and WHIM-truncated zebrafish CXCR4b. Note conservation of serine residues. Arrow marks an identified WHIM truncation mutation. (B) Fluorescence images of human embryonic kidney cells expressing GFP (first column), CXCR4b-GFP (second column), or WHIM-GFP (third column) after incubation with human SDF1 (bottom row) or vehicle control (top row). (C) Schematic of Tol2-MPO:zCXCR4b-WHIM-GFP vector injected to generate WHIM-GFP transgenic lines. (D) Schematic of 3-dpf zebrafish larvae. Boxed region is approximate area magnified in panel E. (E) Fluorescence image of the CHT region of a 3-dpf WHIM-GFP larvae. (F-G) High-magnification image of GFP-expressing neutrophils from the CHT of a MPO:GFP (F) and a WHIM-GFP (G, blow up of box in larva from panel E. Note membrane expression in panel G. Bar = 50 μm (E); 20 μm (B); 10 μm (F-G).
To develop a zebrafish model of WHIM syndrome we generated a stable transgenic line by expressing WHIM-GFP specifically in neutrophils with the use of the MPO promoter (Figure 2C).16 The MPO:WHIM-GFP transgenic line will hereafter be referred to as WHIM-GFP. In accordance with our findings reported with the MPO:GFP transgenic line, we observed GFP+ neutrophils in the CHT in WHIM-GFP larvae (Figure 2D-E; supplemental Figure 2B). WHIM-GFP localized to the cell membrane in individual cells in the CHT (Figure 2G), compared with the diffuse localization of GFP alone (Figure 2F). In contrast to the previously published MPO:GFP16 transgenic, we did not observe ectopic expression of WHIM-GFP in tissues other than hematopoietic cells (supplemental Figure 2B).
Neutrophil development in WHIM-GFP larvae
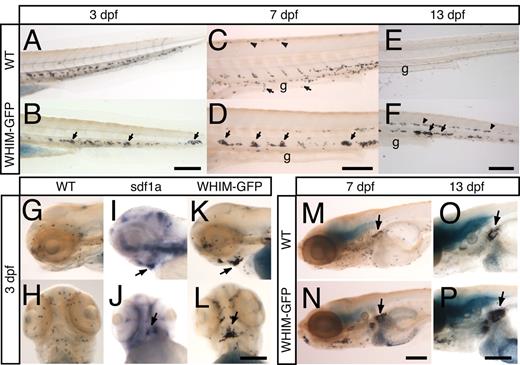
To determine whether expression of the WHIM-GFP receptor affects the distribution of neutrophils during zebrafish development, wild-type or WHIM-GFP larvae were fixed at 2, 3, 7, or 13 dpf and stained with Sudan Black to highlight neutrophils.18 At 3 dpf, we observed normal neutrophil development in the CHT in both wild-type and WHIM-GFP larvae (Figure 3A-B). However, WHIM-GFP larvae showed neutrophil aggregates at 3 dpf (Figure 3B arrows), which were even more apparent at 7 dpf (Figure 3C-D arrows). Neutrophils were not detected ventrally over the gut (Figure 3C arrows and 3D) or along the dorsal ridge of WHIM-GFP larvae as seen in controls (Figure 3C arrowheads and 3D). Strikingly, at 13 dpf, WHIM-GFP larvae showed persistent neutrophil aggregates in the CHT (Figure 3F arrows) and in the midline region (Figure 3F arrowheads), whereas wild-type zebrafish no longer had neutrophils in the CHT (Figure 3E).
Neutrophil development in WHIM-GFP larvae. (A-F) Sudan Black staining in the CHT of wild-type (WT) (A,C,E) or WHIM-GFP (B,D,F) larvae at 3 (A,B), 7 (C,D), and 13 (E,F) dpf. Lateral view, anterior to the left. Arrows point to neutrophils over the gut (C) or in clumps in the CHT (B,D,F). Arrowheads point to neutrophils along the dorsal ridge (C) or midline (F). (G-L) Lateral view (G,I,K) or ventral view (H,J,L) of the head of WT (G-J) or WHIM-GFP (K,L) 3-dpf larvae stained with Sudan Black (G,H,K,L) or for SDF1a expression (I,J) by WISH. Arrows point to area of dark SDF1a expression (I,J) or areas of neutrophil accumulation (K,L) on the ventral side of the head under the jaw. (M-P) Sudan Black staining to show neutrophils in the kidney (arrows) in 7 (M-N) and 13 (O-P) dpf WT (M,O) or WHIM-GFP (N,P) larvae. Lateral view, g indicates gut. Bar = 200 μm (A-P).
Neutrophil development in WHIM-GFP larvae. (A-F) Sudan Black staining in the CHT of wild-type (WT) (A,C,E) or WHIM-GFP (B,D,F) larvae at 3 (A,B), 7 (C,D), and 13 (E,F) dpf. Lateral view, anterior to the left. Arrows point to neutrophils over the gut (C) or in clumps in the CHT (B,D,F). Arrowheads point to neutrophils along the dorsal ridge (C) or midline (F). (G-L) Lateral view (G,I,K) or ventral view (H,J,L) of the head of WT (G-J) or WHIM-GFP (K,L) 3-dpf larvae stained with Sudan Black (G,H,K,L) or for SDF1a expression (I,J) by WISH. Arrows point to area of dark SDF1a expression (I,J) or areas of neutrophil accumulation (K,L) on the ventral side of the head under the jaw. (M-P) Sudan Black staining to show neutrophils in the kidney (arrows) in 7 (M-N) and 13 (O-P) dpf WT (M,O) or WHIM-GFP (N,P) larvae. Lateral view, g indicates gut. Bar = 200 μm (A-P).
Sudan Black staining also showed neutrophil aggregates in the heads of 2- and 3-dpf WHIM-GFP larvae (supplemental Figure 3C-D; Figure 3K and 3L arrows) in contrast to wild-type larvae that show a diffuse distribution of neutrophils (supplemental Figure 3A-B; Figure 3G-H). These neutrophil aggregates persisted on the ventral side of the head at 7 (supplemental Figure 3H arrow) and 13 (supplemental Figure 3J arrow) dpf. Interestingly, less prominent neutrophil aggregates were also observed in the head of transgenic larvae that ectopically express wild-type CXCR4-GFP in neutrophils (supplemental Figure 4A-B). This is consistent with a previous study that reported similar phenotypes with overexpression of wild-type and WHIM-truncated CXCR4 in mouse models.12 These findings indicate that the WHIM truncation is a gain-of-function mutation in vivo.
By 7 dpf, neutrophil development was also apparent in the kidney in wild-type zebrafish (Figure 3M), which is the primary site of adult hematopoiesis. Interestingly, in WHIM-GFP transgenic larvae the hematopoietic tissues of the developing kidney had greater neutrophil accumulation compared with control larvae (Figure 3N arrow). This difference remained prominent at 13 dpf (Figure 3O-P). However, no differences in neutrophil development were observed among wild-type, MPO:GFP, or GFP− siblings of WHIM-GFP larvae. In addition, no differences in apoptosis of neutrophils were observed by staining with terminal deoxynucleotidyl-transferase–mediated deoxyuridine triphosphate nick-end labeling in WHIM-GFP and control larvae (K.B.W., unpublished data, January 2010). Together, these findings suggest that WHIM-GFP larvae have neutrophil aggregates at sites of SDF1a expression, including the CHT and ventral head. Furthermore, WHIM-GFP larvae have neutrophil aggregates in the kidney, the site of neutrophil development in adult zebrafish, reminiscent of the human disorder WHIM syndrome.
CXCR4-SDF1 signaling mediates neutrophil arrest and aggregation in the head
To determine whether neutrophils aggregate in regions of high SDF1a expression in the head, we compared SDF1a expression by WISH with Sudan Black staining. At 2 dpf a ventral view of the head showed 2 bilateral patches of SDF1a expression above the yolk sac (supplemental Figure 3E-F arrows) that converge at 3 dpf to form an area of concentrated SDF1a expression on the ventral side of the head (Figure 3I-J; supplemental Figure 1Gii arrow). The areas of SDF1a expression closely paralleled the location of neutrophil aggregates in the head of WHIM-GFP larvae at both 2 (supplemental Figure 3C-F) and 3 (Figure 3I-L) dpf. In contrast to SDF1a, at 3 dpf SDF1b was expressed on the ventral side of the head (supplemental Figure 1D-E) in areas without neutrophil aggregation, suggesting that neutrophils accumulate in areas with high SDF1a but not SDF1b expression. To confirm this in the context of an individual larva, we performed double WISH with probes for SDF1a (purple) and mpo (red). Indeed, in WHIM-GFP larvae at 3 dpf, we found that mpo expression colocalized with a patch of SDF1a expression on the ventral head (supplemental Figure 3K-L).
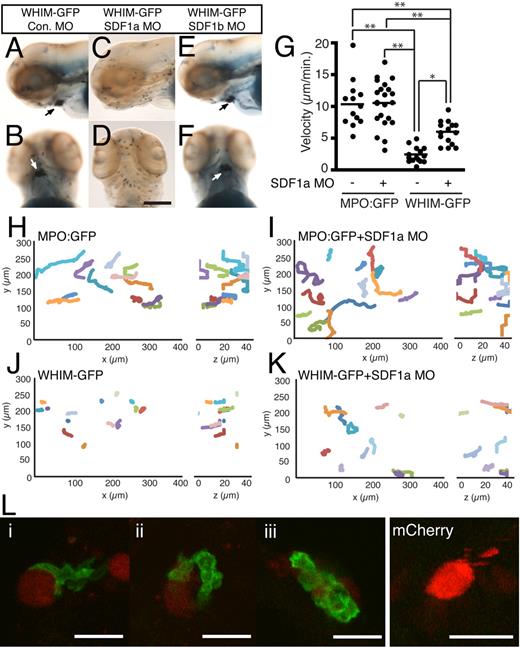
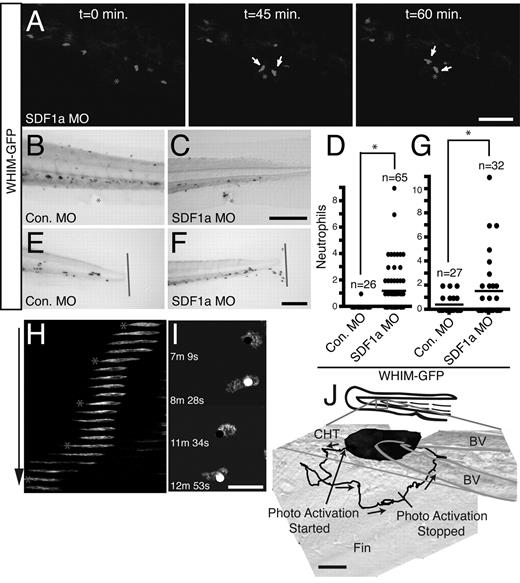
The formation of neutrophil aggregates in areas of high SDF1a expression suggests that WHIM-GFP neutrophils are retained by CXCR4b-SDF1a interactions in vivo. To determine whetherSDF1a mediates neutrophil aggregation in the head, endogenous SDF1a was depleted with MOs. Injection of high concentrations of SDF1a MO (between 1mM and 200μM) resulted in gross morphologic and neutrophil developmental defects (supplemental Figure 5A-B), whereas injection of a lower concentration (110μM) allowed for near normal neutrophil development (supplemental Figure 5C). Injection of the lower concentration of SDF1a (Figure 4C-D) but not SDF1b (Figure 4E-F) or control (Figure 4A-B) MO restored a diffuse distribution of neutrophils in the head, indicating that CXCR4b-SDF1a signaling mediates neutrophil aggregate formation. To characterize how CXCR4b-SDF1a interactions regulate neutrophil aggregation in the head, we monitored neutrophil motility in vivo with the use of live fluorescence microscopy in uninjected and SDF1a morphant MPO:GFP or WHIM-GFP transgenic larvae. Control neutrophils (MPO:GFP) in the head exhibit spontaneous, random migration in zebrafish larvae at 2-3 dpf.28 Live imaging shows rapid polarization and persistent migration of GFP+ control neutrophils in the head at 3 dpf (Figure 4H; supplemental Video 1). Knockdown of SDF1a in MPO:GFP larvae did not affect migration of MPO:GFP neutrophils (Figure 4I; supplemental Video 1). Strikingly, in WHIM-GFP larvae, neutrophils in the head show random protrusions but impaired persistent motility resulting in arrested migration and retention in regions of high SDF1a expression in the head (Figure 4J; supplemental Video 1). Injection of the SDF1a MO partially restored directed neutrophil motility and decreased neutrophil aggregation in WHIM-GFP larvae (Figure 4K; supplemental Video 1), indicating that SDF1a mediates the neutrophil retention signal. The 3-dimensional velocity of neutrophils in WHIM-GFP larvae was significantly impaired compared with control neutrophils (Figure 4G). Injection of the SDF1a MO, however, partially rescued the migration velocity of WHIM-GFP neutrophils (Figure 4G). Taken together these results suggest that CXCR4 WHIM mutations impair persistent neutrophil motility in the head of zebrafish larvae through interactions with endogenously expressed SDF1a.
Neutrophil retention in the head is SDF1a dependent. (A-F) Sudan Black–stained WHIM-GFP larvae injected with either control (A-B), SDF1a (C-D), or SDF1b (E-F) MO. Lateral (A,C,E) or ventral (B,D,F) view of the head at 3 dpf; arrows point to neutrophil accumulation. (G) The mean velocity of tracked neutrophils from the ventral head of uninjected or SDF1a morphant MPO:GFP or WHIM-GFP larvae. **P < .001, *P < .01. (H-K) Neutrophil migration in the ventral head of MPO:GFP (H), SDF1a morphant MPO:GFP (I), WHIM-GFP (J), or SDF1a morphant WHIM-GFP (K) larvae was tracked in 3 dimensions. The tracks are plotted in 3-dimensional space and viewed in the xy-plane (left) or zy-plane (right). Units are in micrometers on each axis. Only tracks of neutrophils that lasted for ≥ 14 minutes and only the first 14 minutes of longer tracks are included. Tracks were taken from supplemental Video 1 with additional tracks in SDF1a morphant larvae from additional videos not shown. (L) WHIM-GFP larvae at 2 or 3 dpf injected with Tol2-CMV:SDF1a-2A-mCherry at the 1-cell stage. Three examples of WHIM-GFP neutrophils (green) in close association with cells expressing SDF1a-2A-mcherry (red) in the body (i), head (ii), and yolk sac (iii). Cells expressing mCherry alone did not recruit WHIM-GFP neutrophils. Bar = 200 μm (A-F); 25 μm (L).
Neutrophil retention in the head is SDF1a dependent. (A-F) Sudan Black–stained WHIM-GFP larvae injected with either control (A-B), SDF1a (C-D), or SDF1b (E-F) MO. Lateral (A,C,E) or ventral (B,D,F) view of the head at 3 dpf; arrows point to neutrophil accumulation. (G) The mean velocity of tracked neutrophils from the ventral head of uninjected or SDF1a morphant MPO:GFP or WHIM-GFP larvae. **P < .001, *P < .01. (H-K) Neutrophil migration in the ventral head of MPO:GFP (H), SDF1a morphant MPO:GFP (I), WHIM-GFP (J), or SDF1a morphant WHIM-GFP (K) larvae was tracked in 3 dimensions. The tracks are plotted in 3-dimensional space and viewed in the xy-plane (left) or zy-plane (right). Units are in micrometers on each axis. Only tracks of neutrophils that lasted for ≥ 14 minutes and only the first 14 minutes of longer tracks are included. Tracks were taken from supplemental Video 1 with additional tracks in SDF1a morphant larvae from additional videos not shown. (L) WHIM-GFP larvae at 2 or 3 dpf injected with Tol2-CMV:SDF1a-2A-mCherry at the 1-cell stage. Three examples of WHIM-GFP neutrophils (green) in close association with cells expressing SDF1a-2A-mcherry (red) in the body (i), head (ii), and yolk sac (iii). Cells expressing mCherry alone did not recruit WHIM-GFP neutrophils. Bar = 200 μm (A-F); 25 μm (L).
To determine whether expression of SDF1a is sufficient to attract WHIM-GFP neutrophils and induce neutrophil aggregation we transiently expressed SDF1a-2A-mCherry in a mosaic fashion in WHIM-GFP larvae. The self-cleaving, viral 2A peptide29 allows for expression of untagged, secreted SDF1a while marking the secreting cells with mCherry. WHIM-GFP neutrophils could be found in close association with SDF1a-expressing cells in a variety of different tissues of the head, body, and yolk sac (Figure 4L), with occasional clusters of WHIM-GFP neutrophils forming in regions with ectopic expression of SDF1a (Figure 4Liii). In contrast, expression of mCherry alone failed to attract WHIM-GFP neutrophils (Figure 4L). Live imaging showed that the association of WHIM-GFP neutrophils and SDF1a-expressing cells was dynamic and persistent, often with long periods of close contact between the cells (supplemental Video 2). Together these results suggest that regionalized expression of SDF1a is necessary and sufficient to form WHIM-GFP neutrophil aggregates.
WHIM-GFP transgenic larvae are neutropenic
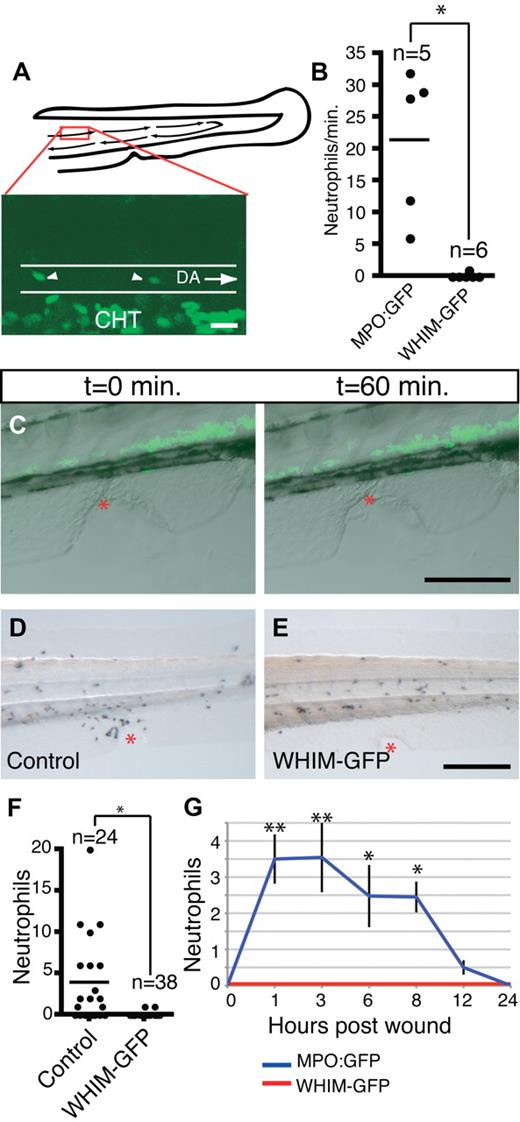
A hallmark of the human disorder, WHIM syndrome, is peripheral neutropenia. To determine whether WHIM-GFP larvae are neutropenic in the vasculature, we used live imaging to scan blood flow for GFP+ cells. In MPO:GFP larvae, neutrophils can be observed in the blood stream by 3 dpf. By quick acquisition of a small section of the dorsal aorta we quantified the number of bright GFP+ cells per minute of blood flow (Figure 5A). In 3- and 4-dpf MPO:GFP transgenic larvae the number of neutrophils in the blood varied from 6 neutrophils/min to 32 neutrophils/min with an average of approximately 21 neutrophils/min of blood flow (Figure 5B; supplemental Video 3). In contrast, GFP+ cells were absent in the blood of WHIM-GFP transgenic larvae at 3-4 dpf (Figure 5B; supplemental Video 3) and 7 dpf (K.B.W., unpublished data, June 2009). These findings show that we have generated a neutropenic model of WHIM syndrome in zebrafish.
WHIM-GFP neutrophils fail to enter the blood stream and respond to wounding in the ventral tailfin. (A) Schematic of the tail of 3-dpf larvae and sample GFP frame from supplemental Video 3; red box is area of dorsal aorta where time-lapse imaging was performed. DA indicates dorsal aorta outlined by white lines, arrows indicate direction of blood flow; CHT, where GFP+ neutrophils not in circulation can be seen. White arrowheads indicate neutrophils in the circulation. (B) Quantification of neutrophils in the blood of MPO:GFP and WHIM-GFP 3-4 dpf transgenic larvae; *P < .01. Each dot represents a separate larva whose blood was analyzed by time-lapse imaging for 1 minute as in supplemental Video 3. (C) Time-lapse imaging of the wound response in WHIM-GFP transgenic larvae (from supplemental Video 4), GFP fluorescence overlaid with differential interference contrast image at indicated time points. (D-E) Sudan Black staining to show neutrophils at wounds in the ventral tailfin in 3-dpf control (D) or WHIM-GFP (E) 2 hours after wound. (F) Quantification of neutrophil recruitment to wounds in fixed larvae as in panels D and E; *P < .001; n = the number of individual larva wounded and counted; control = GFP− siblings of WHIM-GFP larvae. (G) Time course of neutrophil wound recruitment in MPO:GFP and WHIM-GFP transgenic larvae. Error bars = SEM. **P < .001; *P < .01; n = 20-25 larvae at each time point. Bars = 200 μm (C-E); 20 μm (A).
WHIM-GFP neutrophils fail to enter the blood stream and respond to wounding in the ventral tailfin. (A) Schematic of the tail of 3-dpf larvae and sample GFP frame from supplemental Video 3; red box is area of dorsal aorta where time-lapse imaging was performed. DA indicates dorsal aorta outlined by white lines, arrows indicate direction of blood flow; CHT, where GFP+ neutrophils not in circulation can be seen. White arrowheads indicate neutrophils in the circulation. (B) Quantification of neutrophils in the blood of MPO:GFP and WHIM-GFP 3-4 dpf transgenic larvae; *P < .01. Each dot represents a separate larva whose blood was analyzed by time-lapse imaging for 1 minute as in supplemental Video 3. (C) Time-lapse imaging of the wound response in WHIM-GFP transgenic larvae (from supplemental Video 4), GFP fluorescence overlaid with differential interference contrast image at indicated time points. (D-E) Sudan Black staining to show neutrophils at wounds in the ventral tailfin in 3-dpf control (D) or WHIM-GFP (E) 2 hours after wound. (F) Quantification of neutrophil recruitment to wounds in fixed larvae as in panels D and E; *P < .001; n = the number of individual larva wounded and counted; control = GFP− siblings of WHIM-GFP larvae. (G) Time course of neutrophil wound recruitment in MPO:GFP and WHIM-GFP transgenic larvae. Error bars = SEM. **P < .001; *P < .01; n = 20-25 larvae at each time point. Bars = 200 μm (C-E); 20 μm (A).
Impaired chemotaxis of WHIM-GFP neutrophils to wounds
Wounding the fins of zebrafish larvae results in the rapid recruitment of neutrophils,30 and this recruitment can be visualized by live imaging.16 Interestingly, we observed impaired neutrophil chemotaxis to wounds in the ventral tailfin of WHIM-GFP larvae (Figure 5C; supplemental Video 4). To quantify the recruitment defect we used a fixed wounding assay with Sudan Black staining to visualize neutrophils at the wound. At 2 hours after wound there was robust recruitment of neutrophils to the wounds in control larvae with few detectable neutrophils recruited to the wounds of WHIM-GFP larvae (Figure 5D-F). A time course showed impaired neutrophil recruitment to wounds at all time points between 1 and 24 hours after wound (Figure 5G).
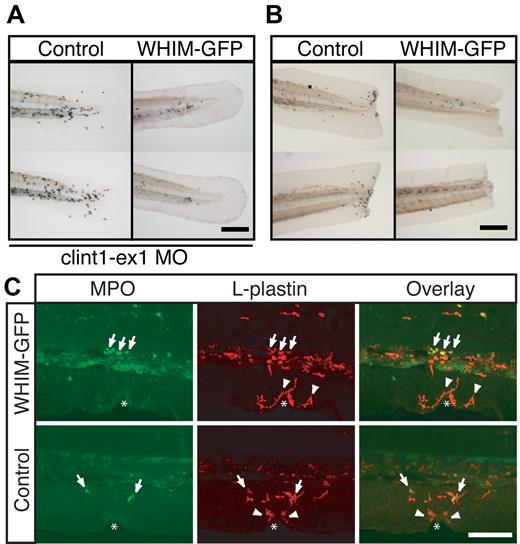
To determine whether WHIM-GFP neutrophils could respond to stronger inflammatory stimuli we examined neutrophil recruitment in response to tail transection and in the context of chronic inflammation. We have previously reported 2 mutant lines, spint1ahi2217 and clint1hi1520, that show epidermal hyperproliferation and robust chronic neutrophil recruitment into the tailfin.19,31 Using a MO targeting clint1 to recapitulate the clint1hi1520 phenotype, we observed neutrophil recruitment into the tailfins of control larvae but not in WHIM-GFP larvae (Figure 6A; supplemental Video 5). Similarly, transient expression of WHIM-GFP in neutrophils of spint1ahi2217 larvae19 impaired neutrophil recruitment to the tailfin (supplemental Video 6). WHIM-GFP neutrophils also failed to respond to tail transections (Figure 6B; supplemental Video 7). Interestingly, although neutrophil chemotaxis to tail transections was impaired, wounding induced increased protrusion and localized motility of WHIM-GFP neutrophils in the CHT (supplemental Video 7) of transgenic larvae. Taken together, these findings suggest that, even in the presence of robust inflammatory stimuli, neutrophils show impaired mobilization and recruitment into inflamed tissues in the WHIM transgenic.
WHIM-GFP neutrophils fail to respond to tail transections or chronic inflammatory signals. (A) Sudan Black staining of tails from Control (left) or WHIM-GFP (right) larvae injected with clint-ex1 MO to induce epidermal hyperproliferation and chronic inflammation in the tail. (B) Sudan Black staining of tail transections in Control (left) or WHIM-GFP (right) larvae. (C) Confocal imaging at wounds in WHIM-GFP (top) or Control (bottom) larvae at 3 dpf immunolabeled with a rabbit antibody to MPO and a fluorescein isothiocyanate–conjugated anti–rabbit Fab fragment (left) followed by a rhodamine-red–conjugated rabbit antibody to L-plastin (middle). Overlapping signals are yellow in the overlay (right). Arrows are MPO+, L-plastin+ neutrophils; arrowheads are MPO−, L-plastin+ macrophages; white * indicates location of the wound. Control = GFP− siblings of WHIM-GFP larvae. Representative images of ≥ 20 larvae in each condition. Bars = 200 μm (A-B); 100 μm (C).
WHIM-GFP neutrophils fail to respond to tail transections or chronic inflammatory signals. (A) Sudan Black staining of tails from Control (left) or WHIM-GFP (right) larvae injected with clint-ex1 MO to induce epidermal hyperproliferation and chronic inflammation in the tail. (B) Sudan Black staining of tail transections in Control (left) or WHIM-GFP (right) larvae. (C) Confocal imaging at wounds in WHIM-GFP (top) or Control (bottom) larvae at 3 dpf immunolabeled with a rabbit antibody to MPO and a fluorescein isothiocyanate–conjugated anti–rabbit Fab fragment (left) followed by a rhodamine-red–conjugated rabbit antibody to L-plastin (middle). Overlapping signals are yellow in the overlay (right). Arrows are MPO+, L-plastin+ neutrophils; arrowheads are MPO−, L-plastin+ macrophages; white * indicates location of the wound. Control = GFP− siblings of WHIM-GFP larvae. Representative images of ≥ 20 larvae in each condition. Bars = 200 μm (A-B); 100 μm (C).
To determine whether ectopic expression of wild-type CXCR4 receptor also affected neutrophil recruitment to wounds, we quantified neutrophil numbers at wounds in transgenic larvae that express wild-type CXCR4-GFP in neutrophils. Although neutrophil recruitment to wounds was reduced with ectopic expression of wild-type CXCR4b (supplemental Figure 4F), neutrophils remained responsive to wounding in contrast to WHIM-GFP larvae (supplemental Figure 4C-F; supplemental Videos 8-9). It is possible that this difference in recruitment may be due to variation in receptor expression levels or alternatively increased activity of the WHIM truncation compared with wild-type receptor. In any case, the findings indicate that the WHIM truncations induce a gain of function in CXCR4 signaling that impairs neutrophil motility in response to inflammatory signals in vivo.
Previously, we reported that larval inflammatory macrophages, a subset of macrophages, express low levels of GFP in MPO:GFP transgenic larvae, are highly phagocytic, have an elongated morphology, respond to wounds in the tailfin, and express L-plastin but not MPO.22 This population of cells will hereafter be referred to as macrophages. In WHIM-GFP larvae, macrophages do not express detectable levels of the WHIM transgene (K.B.W, unpublished data, April 2009). To determine whether macrophage recruitment was also affected in the WHIM-GFP transgenic, we examined macrophage chemotaxis to the wound by quantifying macrophage number with the use of double immunolabeling for MPO and L-plastin as recently reported.22 In control larvae, both L-plastin+/MPO+ neutrophils (Figure 6C bottom row arrows) and L-plastin+/MPO− macrophages (Figure 6C bottom row arrowheads) were recruited to the wounds. In 100% of WHIM-GFP larvae examined, L-plastin+/MPO− macrophages (arrowheads) were present at the wounds, whereas no L-plastin+/ MPO+ neutrophils (arrows) were detected (Figure 6C top row). This is an interesting observation because neutrophils are the first cells recruited to wounds in vivo. Our findings suggest that impaired neutrophil recruitment does not affect the later recruitment of macrophages.
Depletion of SDF1a rescues neutrophil motility in WHIM-GFP larvae
Previous studies have suggested that enhanced CXCR4-SDF1 signaling mediates neutrophil retention in the BM in patients with WHIM syndrome. However, current models are not amenable to directly testing this hypothesis. To determine whetherSDF1a mediates neutrophil retention in the zebrafish model of WHIM syndrome, we depleted endogenous SDF1a with the use of MO. After injection of a control MO, WHIM-GFP neutrophils showed impaired chemotaxis to wounds (Figure 7B) or tail transection (Figure 7E). In contrast, we found that after injection of the SDF1a MO, WHIM-GFP neutrophils showed robust recruitment to wounds (Figure 7A,C; supplemental Video 10) and tail transections (Figure 7F). Quantification showed a significant rescue in the ability of neutrophils to respond to wounding (Figure 7D) or tail transection (Figure 7G) after injection of the SDF1a MO. Taken together, the findings provide the first direct evidence showing that SDF1a is necessary for the neutrophil retention signal induced by CXCR4 WHIM truncations. Moreover, the findings suggest that intrinsic cell motility in the absence of SDF1a is not affected by truncation of CXCR4, but that the mutation increases the sensitivity of neutrophils to endogenous SDF1a, thereby inducing a retention signal that impairs neutrophil recruitment.
Depletion of SDF1a and photoactivation of Rac are sufficient to restore WHIM-GFP neutrophil–directed migration in vivo. (A) Time-lapse imaging (from supplemental Video 10) of GFP fluorescence showing WHIM-GFP neutrophils responding to a wound (*) in the ventral tailfin in a 3-dpf SDF1a morphant larvae. White arrows indicate WHIM-GFP neutrophils. (B-C,E-F) Sudan Black staining of neutrophil response to wounding (* or line) in the ventral tailfin (B-C) or to tail transection (E-F) of 3-dpf WHIM-GFP transgenic larvae injected with control (B,E) or SDF1a (C,F) MO. (D) Quantification of WHIM-GFP neutrophil response in wounded 3-dpf morphant larvae fixed 2 hours after wound as in panels B and C; *P < .01. (G) Quantification of WHIM-GFP neutrophil response to tail transection in 3-dpf morphant larvae fixed 2 hours after transection as in panels E and F; *P < .05. (H-J) Laser stimulation with a 458-nm light induces directed migration of WHIM-GFP neutrophils also expressing mCherry-PA-Rac from the CHT. Repeated photoactivation was used to direct a single WHIM-GFP/mCherry-PA-Rac neutrophil away from the cell aggregate in the CHT into the tailfin. (H) Z-stack images from the indicated time points in supplemental Video 11 were summed into a single 2-dimensional image and then consolidated into a semi–1-dimensional line. Stars indicate time points and position of laser stimulations. (I) Two examples of the WHIM-GFP/mCherry-PA-Rac neutrophil protruding after stimulation from supplemental Video 11. Black circles indicate position of laser stimulation; white circles are included as reference points. (J) Composite differential interference contrast image of the posterior CHT, blood vessels, and tailfin from supplemental Videos 11 and 12 overlaid with the track (black line) of the directed WHIM-GFP/mCherry-PA-Rac neutrophil migration away from and return to the CHT. The starting and stopping points of photoactivation are indicated. Note that after termination of photoactivation the neutrophil immediately returns to the neutrophil aggregate in the CHT. Similar observations were made in 3 different experiments with 3 different larvae. CHT indicates caudal hematopoietic tissue; BV, blood vessel. Arrows indicate direction of migration. Bars = 200 μm (B-D,F); 100 μm (A); 40 μm (J); and 20 μm (I).
Depletion of SDF1a and photoactivation of Rac are sufficient to restore WHIM-GFP neutrophil–directed migration in vivo. (A) Time-lapse imaging (from supplemental Video 10) of GFP fluorescence showing WHIM-GFP neutrophils responding to a wound (*) in the ventral tailfin in a 3-dpf SDF1a morphant larvae. White arrows indicate WHIM-GFP neutrophils. (B-C,E-F) Sudan Black staining of neutrophil response to wounding (* or line) in the ventral tailfin (B-C) or to tail transection (E-F) of 3-dpf WHIM-GFP transgenic larvae injected with control (B,E) or SDF1a (C,F) MO. (D) Quantification of WHIM-GFP neutrophil response in wounded 3-dpf morphant larvae fixed 2 hours after wound as in panels B and C; *P < .01. (G) Quantification of WHIM-GFP neutrophil response to tail transection in 3-dpf morphant larvae fixed 2 hours after transection as in panels E and F; *P < .05. (H-J) Laser stimulation with a 458-nm light induces directed migration of WHIM-GFP neutrophils also expressing mCherry-PA-Rac from the CHT. Repeated photoactivation was used to direct a single WHIM-GFP/mCherry-PA-Rac neutrophil away from the cell aggregate in the CHT into the tailfin. (H) Z-stack images from the indicated time points in supplemental Video 11 were summed into a single 2-dimensional image and then consolidated into a semi–1-dimensional line. Stars indicate time points and position of laser stimulations. (I) Two examples of the WHIM-GFP/mCherry-PA-Rac neutrophil protruding after stimulation from supplemental Video 11. Black circles indicate position of laser stimulation; white circles are included as reference points. (J) Composite differential interference contrast image of the posterior CHT, blood vessels, and tailfin from supplemental Videos 11 and 12 overlaid with the track (black line) of the directed WHIM-GFP/mCherry-PA-Rac neutrophil migration away from and return to the CHT. The starting and stopping points of photoactivation are indicated. Note that after termination of photoactivation the neutrophil immediately returns to the neutrophil aggregate in the CHT. Similar observations were made in 3 different experiments with 3 different larvae. CHT indicates caudal hematopoietic tissue; BV, blood vessel. Arrows indicate direction of migration. Bars = 200 μm (B-D,F); 100 μm (A); 40 μm (J); and 20 μm (I).
Localized activation of Rac is sufficient to direct migration of WHIM-GFP neutrophils
Rac guanosine triphosphatases are critical regulators of SDF1-induced directed migration in hematopoietic stem cells, T cells, cancer cells, and endothelial cells.32-35 Interestingly, previous studies have reported altered SDF1-induced F-actin polymerization in neutrophils from patients with WHIM, suggesting that WHIM neutrophils may have defects in polarized F-actin polymerization.8 To determine whether polarized, Rac-induced, F-actin polymerization is sufficient to rescue the directed migration of neutrophils in WHIM-GFP larvae, we used a genetically encoded Rac1 guanosine triphosphatase, which can be photoactivated reversibly and repeatedly by a 458 nm light,36 and can direct the migration of neutrophils in vivo.28 Photoactivation of Rac at the cell edge of WHIM-GFP neutrophils induced pseudopod protrusion and directed cell migration in WHIM-GFP larvae (Figure 7H-I) similar to control neutrophils.28 Repeated photoactivation of Rac was sufficient to direct the migration of WHIM-GFP neutrophils away from cell aggregates in the CHT (Figure 7J; supplemental Video 11) and into the tailfin (∼ 85μm in this example). After photoactivation was stopped, the neutrophil rapidly migrated back to the cell aggregates in the CHT (Figure 7J; supplemental Video 12). Together the findings suggest that polarized F-actin polymerization induced by Rac activation is sufficient to rescue the directed migration of WHIM-GFP neutrophils in vivo.
Discussion
In this study we report a zebrafish model of a primary immunodeficiency syndrome, WHIM syndrome. We used the optical transparency of zebrafish to visualize how CXCR4-SDF1 signaling affects neutrophil trafficking in vivo. We have shown that expression of WHIM mutations in zebrafish neutrophils induces peripheral neutropenia and neutrophil retention in hematopoietic tissues, reminiscent of the human disorder. Live imaging shows that constitutive CXCR4-SDF1 signaling impairs persistent, directed neutrophil motility, thereby retaining neutrophils in regions of high SDF1a expression and inducing neutrophil aggregates. The neutrophil retention signal induced by WHIM truncation mutations is SDF1a dependent, because depletion of SDF1a with the use of MO restores neutrophil mobilization. The finding that SDF1a is required for neutrophil retention in the WHIM transgenic provides the first in vivo evidence to suggest that constitutive signaling through CXCR4-SDF1 mediates neutrophil retention in hematopoietic tissue contributing to peripheral neutropenia. Taken together, these findings support the utility of the zebrafish system to understand chemokine signaling through CXCR4-SDF1 and to identify putative targets that modify CXCR4-SDF1 functions in vivo.
CXCR4 and SDF1 have been studied during developmental cell migration, including migration of primordial germ cells, development of the lateral line, and movements of endodermal cells in gastrulation.2,37,38 Here, our findings suggest that neutrophils are located in areas where SDF1a mRNA is highly expressed, including the CHT and regions of the head in wild-type larvae. Previous studies have shown that endogenous SDF1a is essential for normal targeting of primordial germ cells in zebrafish.2 In contrast, we found that depletion of endogenous SDF1a mRNA by MO did not affect the targeting of neutrophils in control zebrafish, suggesting that there may be redundancy with other factors that also mediate neutrophil retention in hematopoietic tissues in vivo. However, when higher concentrations of SDF1a MO were used, we found that neutrophil development was impaired with fewer neutrophils present in the CHT, suggesting that endogenous SDF1a may affect targeting of hematopoietic stem cells that mediate neutrophil development in zebrafish, consistent with previous reports in the literature.39
This is, to our knowledge, the first study to visualize the consequences of altered chemokine signaling on neutrophil trafficking in vivo with the use of live imaging. Previous studies have reported that truncations of the CXCR4 receptor that impair receptor internalization do not alter chemotaxis of primordial germ cells, but rather affect the precision of migration by inducing prolonged “runs” and less tumbling-type migration in vivo.15 In contrast to the findings with primordial germ cell migration, we found that neutrophils in the WHIM transgenic displayed prominent random membrane protrusions but impaired persistent motility in vivo, resulting in retention within areas of high SDF1a expression. Our findings indicate that truncation of the C-terminal tail increases CXCR4 sensitivity to endogenous SDF1, supporting previous reports that suggest WHIM truncations block proper internalization and desensitization of the CXCR4 receptor.8,9,13 Taken together, the findings suggest that constitutive CXCR4-SDF1 signaling induces neutrophil stopping or retention within areas of concentrated SDF1a, including hematopoietic tissues, thereby impairing neutrophil trafficking into the vasculature or to tissue wounds.
There has been substantial recent interest in defining mechanisms that stop cell motility and contribute to retention within tissues. Perhaps the best studied example of stop signals has been in the context of T-cell motility stopping in response to contact with antigen-presenting cells with cognate antigen.40 Neutrophils have also been reported to display stopped migration in response to specific factors, including tumor necrosis factor-α.41 Stop signals have also been implicated in chemokine-mediated signaling in vitro. For example, exposure of neutrophils to one chemokine can impair the migration to other chemokines in vitro.42 Here, we demonstrate the effects of competing signals on neutrophil motility in vivo. Constitutive signaling through CXCR4-SDF1 impairs neutrophil recruitment to wounds despite competing gradients of hydrogen peroxide produced at the wound.43 Interestingly, polarized Rac activation was sufficient to overcome this retention signal and induce directed migration of WHIM-GFP neutrophils. Taken together, our findings suggest that neutrophil retention signals probably play an essential role in affecting neutrophil trafficking in vivo.
Zebrafish provide a powerful system to model human disease and to allow unique insight into disease pathogenesis because events can be visualized in vivo by real-time imaging. This has been shown in studies observing leukocyte trafficking in the context of chronic inflammation mutant lines,19,31,44 bacterial infection,45 and during granuloma formation in zebrafish mycobacterial infection.46 In addition, depletion of WASp, the protein mutated in the immune disorder Wiskott-Aldrich Syndrome, has shown defects in wound inflammation and clot formation.47 The zebrafish model of WHIM syndrome presented here provides novel insight into the pathogenesis of neutropenia in patients with WHIM syndrome. The findings show that the WHIM truncations induce a gain of CXCR4 function that requires the presence of SDF1a. Human studies and mouse models of WHIM syndrome supported the hypothesis that the WHIM mutation induced neutrophil retention in the BM, causing neutropenia. We now provide direct evidence that WHIM-induced neutrophil retention is mediated by altered signaling that can be rescued by depletion of SDF1a and blocking CXCR4-SDF1 signaling. Patients with WHIM are commonly treated with granulocyte colony-stimulating factor (G-CSF), which is thought to mobilize neutrophils from the BM by down-regulating expression of CXCR4 on neutrophils and reducing SDF1 expression in the BM.48,49 Because the zebrafish homolog of G-CSF has been identified and characterized,50 the zebrafish model of WHIM syndrome provides an ideal system to study how G-CSF affects neutrophil trafficking in vivo in WHIM syndrome.
Here, we used the optical transparency of zebrafish to visualize how CXCR4-SDF1 signaling affects neutrophil trafficking in vivo. Our work has demonstrated that constitutive signaling through the CXCR4 receptor induces neutrophil retention in hematopoietic tissue that impairs neutrophil motility and recruitment to inflamed tissues. The neutrophil retention signal is mediated by CXCR4-SDF1 signaling, because depletion of SDF1 restores neutrophil motility and wound recruitment. We propose that the WHIM transgenic zebrafish will provide a powerful in vivo tool to screen for agents that modify CXCR4-SDF1 signaling and may therefore have therapeutic potential.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Erez Raz for generous gifts of plasmids; J.R. Mathias, M.E. Dodd, Q. Deng, P.-Y. Lam, and E.A. Harvie for zebrafish feeding and maintenance; A.J. Wiemer, S.A. Wernimont, K.T. Chan, T.W. Starnes, and C.L. Cortesio for critical reading of the manuscript; and J.J. TeSlaa for help with sectioning.
This work was supported by the National Institutes of Health (grant GM074827; A.H.).
National Institutes of Health
Authorship
Contribution: K.B.W. designed and performed experiments, analyzed data, and wrote the paper; J.M.G., J.C.S., and S.K.Y. performed experiments; and A.H. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anna Huttenlocher, University of Wisconsin-Madison, 1550 Linden Dr, Madison, WI 53706; e-mail: huttenlocher@wisc.edu.
References
Supplemental data
Note the migratory Dendra2 expressing neutrophils in the tailfin (left) compared to the absence of WHIM-GFP neutrophils in the tailfin (center). The right panel shows WHIM-GFP neutrophils in the CHT of the same larvae shown in the center panel. Images were acquired every 1 min. and fluorescence Z-series images were stacked and overlaid with DIC.Additional supplemental videos can be found here.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal