Abstract
The functional activity of the organic cation transporter 1 (OCT-1) protein in chronic myeloid leukemia (CML) mononuclear cells (MNCs) is highly predictive of molecular response in imatinib treated patients. Here we investigate whether the MNC OCT-1 activity (OA) provides a surrogate indicator of effective targeting of the more immature CD34+ cells. While confirming our previous findings that high MNC OA is significantly associated with the achievement of major molecular response (MMR; P = .017), the present studies found no relationship between high CD34+ OA and the achievement of MMR. Furthermore, no correlation was found between the MNC OA and the CD34+ OA in matched CML samples. These results suggest that the predictive value of the MNC OA may primarily reflect the effective targeting and subsequent reduction of mature CML cells. Therefore kinase inhibition in these mature cells, and not the CD34+ cells, may be the key determinant of response in CML.
Introduction
Despite excellent responses to imatinib treatment for the majority of chronic-phase (CP) chronic myeloid leukemia (CML) patients,1 25%-35% of patients display resistance to imatinib and fail therapy.2 High-dose imatinib upfront has been employed in an attempt to improve outcomes, however results are conflicting.3,4 To this end, many studies have focused on identifying prognostic indicators of response5,6 to enable preferential targeting of those patients at risk. In particular, primitive CD34+ CML cells, believed to be most important for complete disease eradication,7 have been investigated.8
The polyspecific organic cation transporter 1 (OCT-1) is the major active influx pump for imatinib in CML cells.9,10 Several studies have identified a relationship between OCT-1 mRNA levels and cytogenetic responses to imatinib treatment.11,12 We have demonstrated that the functional OCT-1 activity (OA) in mononuclear cells (MNCs) from CP-CML patients is highly variable (median: 7.2 ng/200 000 cells, range: 0-31.2), and predicts for a patient's subsequent molecular response, event-free and transformation-free survival.13,14 Of significance, for patients on lower doses of imatinib (below 600 mg) having a low MNC OA is associated with poorer responses, with 73% of these patients failing to achieve a major molecular response (MMR) by 5 years of imatinib treatment.14
We have recently found the OA in CML CD34+ cells to be significantly lower than that in mature CML cells, however a 10-fold variation in CD34+ OA remains (median: 4.0 ng/200 000 cells, range: 0-9.6).15 Given the strong prognostic value of MNC OA for molecular response and survival, this study sought to ascertain whether a patient's MNC OA was related to the OA in their CD34+ cells and whether MNC OA provides a surrogate indicator for efficient targeting of the primitive leukemic population and hence the depth of response.
Methods
Blood and marrow were collected with informed consent, in accordance with the Declaration of Helsinki, from untreated CP-CML patients. Ethical approval for this study was obtained from the Royal Adelaide Hospital (RAH) ethics committee. MNCs were isolated by density gradient centrifugation. CD34+ cells were isolated using magnetic-activated cell sorting positive selection. Imatinib and [14C]-imatinib were provided by Novartis and the OA was determined using the intracellular uptake and retention assay as previously described.10,13 Patients were treated with 400 mg, 600 mg, or 800 mg of imatinib and achievement of MMR was assessed by real-time Quantitative polymerase chain reaction as previously described.16 Statistical comparisons were performed using the Student t, Mann-Whitney Rank Sum, Pearson Product Moment, and Fisher Exact tests as appropriate and as indicated in the figure legends.
Results and discussion
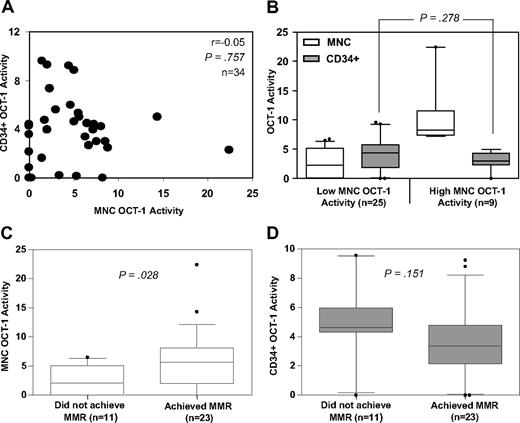
We have clearly demonstrated that a high OA, measured in a patient's MNCs at diagnosis, is a strong predictor of good molecular response, event-free and transformation-free survival in response to imatinib treatment.13,14 These findings raise the following questions: Why is OA (and hence imatinib accumulation) in CML MNCs so predictive of response? Is our OA assay in MNCs a surrogate indicator for how efficiently the primitive population is being targeted? To address this possibility, we examined the relationship between the OA in a patient's mature MNCs and the OA in their primitive CD34+ cells. In this cohort of 34 patients the median MNC and CD34+ OA was 4.8 ng/200 000 cells and 4.0 ng/200 000 cells, respectively. No correlation was observed between the OA in MNCs and in CD34+ cells (r = −0.05, P = .757, Figure 1A). We then divided patients into low (n = 25) and high (n = 9) groups based on their MNC OA (∼ 7.2 ng/200 000 cells13 ). We found no significant difference in CD34+ OA between these 2 groups (P = .278, Figure 1B). Hence, having a high MNC OA did not correlate with having a high CD34+ OA.
Relationship between MNC OCT-1 activity, CD34+ OCT-1 activity and achievement of major molecular response. (A) MNC OCT-1 activity and CD34+ OCT-1 activity was measured in 34 CP-CML patients. In these matched samples no correlation was observed between MNC OCT-1 activity and CD34+ OCT-1 activity. The Pearson Product Moment was used to assess the correlation. (B) Patients were divided into high and low MNC OCT-1 activity groups (∼ 7.2 ng/200 000 cells13 ). Their corresponding CD34+ OCT-1 Activities were compared. No difference in CD34+ OCT-1 activity was seen between the 2 groups. The median CD34+ OCT-1 activity in the low MNC group was 4.38 ng/200 000 cells and the median CD34+ OCT-1 activity in the high MNC group was 3.0 ng/200 000 cells. Therefore, a high MNC OCT-1 activity is not indicative of whether a patient will have a high CD34+ OCT-1 activity. P values were calculated using the Student t test. (C) Patients were grouped according to the achievement of MMR by 12 months of imatinib treatment and the MNC OCT-1 activity was compared between the 2 groups. The median MNC OCT-1 activity in patients who achieved MMR was 5.64 ng/200 000 cells, whereas for patients who did not achieve MMR the median MNC OCT-1 activity was significantly lower at 2.05 ng/200 000 cells. P values were calculated using the Mann-Whitney Rank Sum. (D) Patients were grouped according to the achievement of MMR by 12 months of imatinib treatment and the CD34+ OCT-1 activity was compared between the 2 groups. There was no significant difference in the CD34+ OCT-1 activity between patients who did and did not achieve MMR. The median CD34+ OCT-1 activity in patients who achieved MMR was 3.36 ng/200 000 cells and the median CD34+ OCT-1 activity in patients who did not achieve MMR was 4.62 ng/200 000 cells. P values were calculated using the Student t test.
Relationship between MNC OCT-1 activity, CD34+ OCT-1 activity and achievement of major molecular response. (A) MNC OCT-1 activity and CD34+ OCT-1 activity was measured in 34 CP-CML patients. In these matched samples no correlation was observed between MNC OCT-1 activity and CD34+ OCT-1 activity. The Pearson Product Moment was used to assess the correlation. (B) Patients were divided into high and low MNC OCT-1 activity groups (∼ 7.2 ng/200 000 cells13 ). Their corresponding CD34+ OCT-1 Activities were compared. No difference in CD34+ OCT-1 activity was seen between the 2 groups. The median CD34+ OCT-1 activity in the low MNC group was 4.38 ng/200 000 cells and the median CD34+ OCT-1 activity in the high MNC group was 3.0 ng/200 000 cells. Therefore, a high MNC OCT-1 activity is not indicative of whether a patient will have a high CD34+ OCT-1 activity. P values were calculated using the Student t test. (C) Patients were grouped according to the achievement of MMR by 12 months of imatinib treatment and the MNC OCT-1 activity was compared between the 2 groups. The median MNC OCT-1 activity in patients who achieved MMR was 5.64 ng/200 000 cells, whereas for patients who did not achieve MMR the median MNC OCT-1 activity was significantly lower at 2.05 ng/200 000 cells. P values were calculated using the Mann-Whitney Rank Sum. (D) Patients were grouped according to the achievement of MMR by 12 months of imatinib treatment and the CD34+ OCT-1 activity was compared between the 2 groups. There was no significant difference in the CD34+ OCT-1 activity between patients who did and did not achieve MMR. The median CD34+ OCT-1 activity in patients who achieved MMR was 3.36 ng/200 000 cells and the median CD34+ OCT-1 activity in patients who did not achieve MMR was 4.62 ng/200 000 cells. P values were calculated using the Student t test.
We next examined whether CML CD34+ OA is predictive of molecular response to imatinib treatment. We anticipated that CD34+ OA would be an equivalent, if not better predictor of response than MNC OA because effective targeting of primitive cells would be considered to be a prerequisite for a deep molecular response. The OA in MNCs and CD34+ cells from these patients were assessed against their molecular response over the first 12 months of imatinib therapy. Patients were grouped into those who had achieved MMR (n = 23) or not (n = 11) by 12 months. Those patients who achieved MMR had a significantly higher MNC OA compared with those who did not achieve MMR (P = .028, Figure 1C). Surprisingly, there was no significant difference in the CD34+ OA between patients who achieved MMR and those who did not (P = .151, Figure 1D). Furthermore, and in support of our previous findings,13,14 Fisher Exact analysis revealed a significant association between MNC OA and the achievement of MMR. One hundred percent of patients with a high MNC OA achieved MMR by 12 months of treatment in comparison to 56% with low MNC OA (P = .017, Table 1). Dividing patients about the median CD34+ OA of 4.0 ng/200 000 cells revealed a converse association with the achievement of MMR. Forty-seven percent of patients with a high CD34+ OA achieved MMR by 12 months in comparison to 88% with low CD34+ OA (P = .026, Table 1). Therefore, low OA in patient's CD34+ cells appears to be associated with better molecular responses.
OCT-1 activity and molecular response
| . | Achieved MMR (% of n) . | Did not achieve MMR (% of n) . |
|---|---|---|
| MNC (P = .017) | ||
| High OA (n = 9) | 9 (100%) | 0 (0%) |
| Low OA (n = 25) | 14 (56%) | 11 (44%) |
| CD34+ (P = .026) | ||
| High OA (n = 17) | 8 (47%) | 9 (53%) |
| Low OA (n = 17) | 15 (88%) | 2 (12%) |
| . | Achieved MMR (% of n) . | Did not achieve MMR (% of n) . |
|---|---|---|
| MNC (P = .017) | ||
| High OA (n = 9) | 9 (100%) | 0 (0%) |
| Low OA (n = 25) | 14 (56%) | 11 (44%) |
| CD34+ (P = .026) | ||
| High OA (n = 17) | 8 (47%) | 9 (53%) |
| Low OA (n = 17) | 15 (88%) | 2 (12%) |
Shown are the number of patients who did and did not achieve a major molecular response (MMR) over the first 12 months of imatinib therapy. P values were calculated using the Fisher Exact test.
In combination, these data suggest that MNC OA is not correlated with CD34+ OA, and only high MNC OA is predictive of favorable patient responses to imatinib treatment. This observation raises an important question: Why is the OA for imatinib in end-stage CML cells so predictive of response when it is presumably the fate of primitive leukemic cells that is the determinant of the long-term risk of disease progression and drug resistance?7,17,18
Several studies have attributed the resistance of primitive CML cells to imatinib to the autocrine production of cytokines such as interleukin-3 (IL-3),18,19 granulocyte colony-stimulating factor (G-CSF),18,19 and granulocyte-macrophage colony-stimulating factor (GM-CSF).20 This has been confirmed in cell line studies where BCR-ABL expression initiates factor independence through secretion of IL-3 and GM-CSF.21 However, resistance through paracrine mechanisms is not as well understood. Studies from our laboratory have shown that mature CML MNCs secrete GM-CSF and coculturing CML CD34+ cells with mature MNCs increased their proliferation and their survival in the presence of imatinib.22 Furthermore, serum levels of GM-CSF and G-CSF have been shown to be elevated in vivo in CML patients compared with normal.23 Similarly, mice transplanted with BCR-ABL transduced bone marrow display increased transcripts and serum levels of IL-3 and GM-CSF.24 From this, we speculate that a rapid depletion of mature CML cells by imatinib (as predicted by the OA assay) may deprive CD34+ cells of essential cytokines normally produced by the mature leukemic population. This cytokine-depleted environment may remove the proliferative advantage of leukemic hematopoiesis, facilitating the regrowth of the residual nonleukemic hematopoietic cells that will be reflected in deeper molecular responses.
In conclusion CD34+ OA is unrelated to MNC OA and high OA in this primitive compartment is not associated with good molecular responses to imatinib treatment. This suggests that the depletion of mature CML cells may facilitate a lower risk of drug resistance and disease progression, possibly due to depletion of essential cytokines. The exciting and novel implication of these findings is that direct targeting of this primitive population may not be essential for achievement of early and deep molecular responses, and the low risk of progression associated with these responses in CP-CML patients treated with tyrosine kinase inhibitors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Many thanks to Sasha Wheeler and Stephanie Arbon for their assistance in compiling the molecular response data. We are grateful to Thomas Sullivan for his assistance in the statistical analyses.
We acknowledge the support of Novartis Pharmaceuticals for providing the imatinib and [14C]-imatinib.
J.E. is supported by a PhD scholarship from the Leukemia Foundation of Australia.
Authorship
Contribution: J.R.E., D.L.W., and T.P.H. designed the research; J.R.E., A.F., and V.S. performed the research; J.R.E. analyzed the data; and J.R.E., A.Z., D.L.W., and T.P.H. prepared the manuscript.
Conflict-of-interest disclosure: T.P.H. and D.L.W. receive honoraria and grant funding from Novartis Pharmaceuticals and are members of advisory boards for Novartis. A.Z. receives grant funding from Novartis Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Dr Deborah L. White, Division of Haematology, SA Pathology (RAH Campus), Frome Rd, Adelaide, Australia; e-mail: deborah.white@health.sa.gov.au.