Abstract
Interferon regulatory factor 4 (IRF-4) plays important functions in B- and T-cell development and immune response regulation and was originally identified as the product of a proto-oncogene involved in chromosomal translocations in multiple myeloma. Although IRF-4 is expressed in myeloid cells, its function in that lineage is not known. The closely related family member IRF-8 is a critical regulator of myelopoiesis, which when deleted in mice results in a syndrome highly similar to human chronic myelogenous leukemia. In early lymphoid development, we have shown previously that IRF-4 and IRF-8 can function redundantly. We therefore investigated the effects of a combined loss of IRF-4 and IRF-8 on hematologic tumorigenesis. We found that mice deficient in both IRF-4 and IRF-8 develop from a very early age a more aggressive chronic myelogenous leukemia-like disease than mice deficient in IRF-8 alone, correlating with a greater expansion of granulocyte-monocyte progenitors. Although these results demonstrate, for the first time, that IRF-4 can function as tumor suppressor in myeloid cells, interestingly, all mice deficient in both IRF-4 and IRF-8 eventually develop and die of a B-lymphoblastic leukemia/lymphoma. Combined losses of IRF-4 and IRF-8 therefore can cooperate in the development of both myeloid and lymphoid tumors.
Introduction
Interferon regulatory factor 4 (IRF; also known as Pip, LSIRF, ICSAT, and MUM1) is a transcription factor that plays important roles in B- and T-cell development and immune response regulation.1,2 It was also originally identified as the product of a proto-oncogene involved in chromosomal translocations in multiple myeloma.3 Its ability to transform lymphocytes in vitro and its abnormal expression patterns in B-cell and T-cell lymphomas and leukemias are well established.4,5 In addition to lymphoid cells, IRF-4 expression has been found in macrophages.6,7 However, its function in the myeloid lineage is not well characterized.
The closely related family member IRF-8 (also known as interferon consensus sequence binding protein) has more clearly defined functions in the myeloid lineage,8 and its loss has been strongly implicated in the pathogenesis of chronic myelogenous leukemia (CML). CML is a myeloproliferative disease characterized by the underlying t(9;22)(q34;q11) reciprocal translocation known as the Philadelphia chromosome, which leads to creation and expression of the fusion gene product BCR/ABL, a constitutively active tyrosine kinase.9,10 The disease has a relatively mild chronic phase, an accelerated myeloproliferative phase, and finally a transformation to blast crisis, which is rapidly fatal without aggressive myeloablative treatment. Before the introduction in recent years of tyrosine kinase inhibitors, such as imatinib myselate, the standard treatment for the chronic phase of CML was interferon-α (IFN-α), which induces a complete or nearly complete cytogenetic response in 10% to 30% of patients. Several lines of evidence show that IRF-8 functions as a myeloid tumor suppressor and a mediator of IFN-α treatment of CML. A germline null mutation of IRF-8 results in animals that spontaneously develop a CML-like disease at 10 to 16 weeks of age.11 Approximately one-third of IRF-8–deficient mice become moribund by 50 weeks of age, showing a blast crisis involving both myeloid and lymphoid lineages.11 In BXH-2 mice, a loss-of-function mutation in IRF-8 was shown to be the underlying cause of a myeloproliferative syndrome that eventually progresses to a CML-like disease after the accumulation of additional mutations.12 In addition, lower expression levels of IRF-8 in human CML patients are correlated with a higher burden of pretreatment risk factors and less likelihood of response to treatment with IFN-α.13 We have shown that IRF-8 is down-regulated in a BCR/ABL-induced murine model14 of CML and that forced overexpression of IRF-8 in this model represses the resulting myeloproliferative disorder (MPD) and prolongs survival.15 IRF-8 also represses the mitogenic activity of BCR/ABL in transformed myeloid cells grown in culture.16 Subsequent studies suggest that the mechanism of IRF-8's tumor suppression is in part down-regulation of expression of Bcl-2 and up-regulation of the c-Myc inhibitors Blimp1 and METs.17
Similar to IRF-8 and in contrast to its oncogenic activity in lymphoid cells, IRF-4 expression was shown to be down-regulated in patients with CML but restored in response to treatment with IFN-α. Patients with higher IRF-4 expression had better responses to IFN-α therapy.18 This effect was seen initially primarily in the T cells of CML patients, but a more recent study by the same group found impaired IRF-4 expression in a variety of leukemic cell lines.19 Although these findings suggest that lost expression of IRF-4 may be an important factor in CML pathogenesis and the failure of some patients to respond to IFN-α, its ability to function as a myeloid tumor suppressor has not been demonstrated. Interestingly, despite the many similarities in structure and function between IRF-4 and IRF-8, the described phenotype of IRF-4–deficient mice is of deficient B and T lymphocyte function20 and failure of development of certain dendritic cell subsets,21 in contrast to the primarily myeloid phenotype seen in IRF-8-deficient animals.
In B-cell development, we have shown that IRF-4 and IRF-8 function redundantly at the pre-B cell development, IRF-4/8 double, but not single, deficiencies lead to blocking the transition from large, cycling pre-B cells to small, resting pre-B cells.22 In the current study, we investigated whether IRF-4 and IRF-8 may also have overlapping function in the myeloid system. We found that mice lacking both IRF-4 and IRF-8 develop, from a very early age, a much more aggressive CML-like disease than those lacking IRF-8 alone. All mice deficient in both IRF-4 and IRF-8 eventually develop and die of a B-lymphoblastic leukemia/lymphoma by approximately 25 weeks of age. The results demonstrate that deficiencies of IRF-4 and IRF-8 can cooperate in the development of both myeloid and lymphoid tumors.
Methods
Knockout mice and characterization
IRF-4 knockout (KO), IRF-8 KO, and IRF-4/8 DKO mice were bred and genotyped as described previously.23 Peripheral blood (PB) was obtained from tails for blood smears, white blood cell (WBC) counts, and flow cytometric analysis. Smears were subjected to Wright-Giemsa staining. WBC counts were obtained on hemacytometer under light microscopy after diluting PB in Turks solution (2% acetic acid, 0.01% methylene blue). Spleens were obtained for flow cytometric analysis and Hoechst and eosin staining after paraffin embedding using standard protocols. Bone marrow (BM) cells were obtained by aspiration from the femurs and tibias of subject animals and subjected to flow cytometric analysis.
Mice used in this project were housed in the Association for Assessment and Accreditation of Laboratory Animal Care International–accredited Foster Animal Research Facility at Brandeis University. All experiments involving mice are approved by the Institutional Animal Care and Use Committee of Brandeis University.
Ex vivo analysis of progenitor cells
BM cells were lineage-depleted using biotinylated antibodies (BD Pharmingen) against CD3, CD4, CD8, CD19, IgM, Gr1, B220, Ter119, and BD IMag particles (BD Biosciences). Depleted cells were plated into 24-well plates (3 × 105 cells/well), grown in Dulbecco modified Eagle medium containing 10% fetal bovine serum, penicillin/streptomycin, and 2-mercaptoethanol. Granulocyte-macrophage colony-stimulating factor (GM-CSF) was added to a concentration of 0.01, 0.1, and 1 ng/mL. For the proliferation assay, 10μM 5-bromo-2-deoxyuridine (BrdU) was added for 45 minutes before fixing cells. After staining with allophycocyanin (APC)–conjugated anti-BrdU antibody, flow cytometric analysis was performed on a FACSCalibur Flow Cytometer (BD Biosciences).
Flow cytometric analysis
Standard protocols for antibody staining of cell surface proteins were followed.23 Cells from PB or BM were treated with ammonium chloride (ACK) solution (0.15M NH4Cl, 1.0mM KHCO3, 0.1mM Na2 ethylenediaminetetraacetic acid, pH 7.3) to lyse red blood cells and then resuspended in staining buffer (phosphate-buffered saline, 1% fetal bovine serum, 0.1% sodium azide) and blocked with antimouse CD16-CD32 (Fc block) (BD Pharmingen). Cells were then stained with the following antibodies (BD Biosciences PharMingen): APC-conjugated Mac1 (M1/70) (APC-Mac1), phycoerythrin-Gr1 (RB6-8C5), APC-B220, phycoerythrin-CD19, phycoerythrin-CD43, APC-Thy1.2, phycoerythrin-CD3, APC-c-kit, and phycoerythrin-Ter119. After staining, cells were washed with phosphate-buffered saline and resuspended in staining buffer containing propidium iodide to label dead cells. Flow cytometry was performed on a FACSCalibur Flow Cytometer (BD Biosciences), and data were analyzed with FlowJo software Volume 4.6 (TreeStar). For analysis of myeloid progenitors, BM cells were incubated for 30 minutes on ice with biotinylated lineage-specific antibodies, including CD3, CD4, CD8, CD19, IgM, Gr1, B220, Ter119, and CD127 (IL-7R) followed by staining with fluorescein isothiocyanate–conjugated CD34, phycoerythrin-CD16/32, APC-Cy7-CD117, APC-Sca1, and PerCP-Cy5.5-streptavidin. Flow cytometric analysis was done using a FACSAria Flow Cytometer (BD Biosciences).
Results
IRF-4/8 DKO mice develop an aggressive CML-like disease much earlier than IRF-8 KO mice
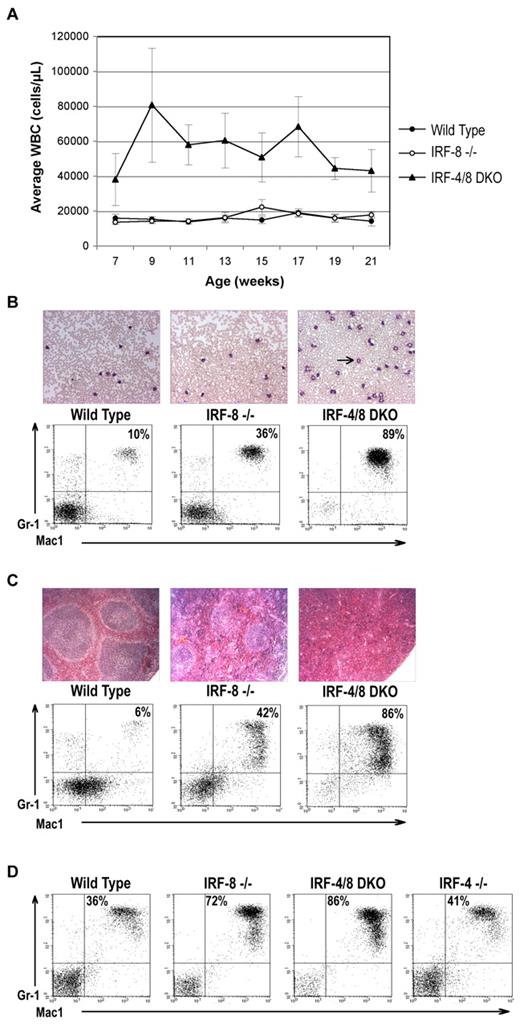
To determine whether IRF-4 and IRF-8 have overlapping functions in myelopoiesis, we compared the myeloid phenotype of IRF-8 KO mice with that of IRF-4/8 DKO mice. IRF-4 KO mice were not included in the experiment because they do not develop an MPD phenotype or other obvious abnormalities in myeloid development20 (and data not shown). From 7 weeks of age, the DKO mice showed a much more aggressive MPD phenotype than IRF-8 KO mice. The WBC counts of DKO mice range from 40 000 to 80 000 cells/μL compared with 15 000 to 20 000 cells/μL for IRF-8 KO animals (Figure 1A). Failure of the IRF-8 KO mice to show a difference with wild-type animals at this early stage is consistent with the previously described phenotype of the IRF-8 KO animals, in which PB changes are seen only well after the development of the CML-like disease in BM and lymphoid organs.11 Even in IRF-8 KO mice with fully developed MPD, the WBC counts were only moderately elevated11 (and data not shown).
IRF-4 deficiency exacerbates the development of CML-like MPD in IRF-8 KO mice. (A) Average WBC counts in wild-type, IRF-8 KO, and IRF-4/8 DKO mice over the course of a 21-week experiment. (B) Representative PB smears and FACS profiles of peripheral WBCs obtained from animals at the age of 9 weeks. IRF-4/8 DKO animals have expansion of cells with granulocytic morphology (arrow) and staining double positive for the cell surface markers Mac-1 and Gr-1. (C) Hematoxylin and eosin–stained spleens isolated from animals 5 to 6 months of age show complete effacement of the normal microarchitecture by infiltrating granulocytic cells in IRF-4/8 DKO animals, with relative sparing in IRF-8 KO mice. Relative proportions of Mac-1+/Gr-1+ cells are shown in the accompanying FACS analyses. Image acquisition informaton: Zeiss Axioskop 40 microscope; blood smears: 20×/0.45 Ph2; spleens: 10×/0.25; Zeiss AxioCam MRc; AxioVision Red 4.6 software; PowerPoint and Adobe Photoshop 7.0. (D) Representative FACS analysis of BM cells obtained from animals 5 to 6 months of age. The IRF-4/8 DKO animal shows massive expansion of Mac1+ and Gr1+ granulocytes.
IRF-4 deficiency exacerbates the development of CML-like MPD in IRF-8 KO mice. (A) Average WBC counts in wild-type, IRF-8 KO, and IRF-4/8 DKO mice over the course of a 21-week experiment. (B) Representative PB smears and FACS profiles of peripheral WBCs obtained from animals at the age of 9 weeks. IRF-4/8 DKO animals have expansion of cells with granulocytic morphology (arrow) and staining double positive for the cell surface markers Mac-1 and Gr-1. (C) Hematoxylin and eosin–stained spleens isolated from animals 5 to 6 months of age show complete effacement of the normal microarchitecture by infiltrating granulocytic cells in IRF-4/8 DKO animals, with relative sparing in IRF-8 KO mice. Relative proportions of Mac-1+/Gr-1+ cells are shown in the accompanying FACS analyses. Image acquisition informaton: Zeiss Axioskop 40 microscope; blood smears: 20×/0.45 Ph2; spleens: 10×/0.25; Zeiss AxioCam MRc; AxioVision Red 4.6 software; PowerPoint and Adobe Photoshop 7.0. (D) Representative FACS analysis of BM cells obtained from animals 5 to 6 months of age. The IRF-4/8 DKO animal shows massive expansion of Mac1+ and Gr1+ granulocytes.
PB smears and fluorescence-activated cell sorter (FACS) analyses show that the WBC increase in DKO animals is the result of a massive expansion of granulocytic cells (Figure 1B). In addition, histopathologic and FACS analyses show that, by 15 weeks of age, the spleens (Figure 1C), BM (Figure 1D), and lymph nodes (data not shown) of DKO animals were infiltrated by large numbers of granulocytes, with complete effacement of the normal microarchitecture. Age-matched IRF-8 KO mice showed invasion to a lesser degree and preservation of many of the normal architectural features (Figure 1C-D; and data not shown). These data demonstrate that IRF-4 functions as a tumor suppressor in myeloid cells and negatively regulates myeloid cell expansion.
IRF-4/8 DKO BM progenitors have a greater proliferative capacity than WT or single KOs
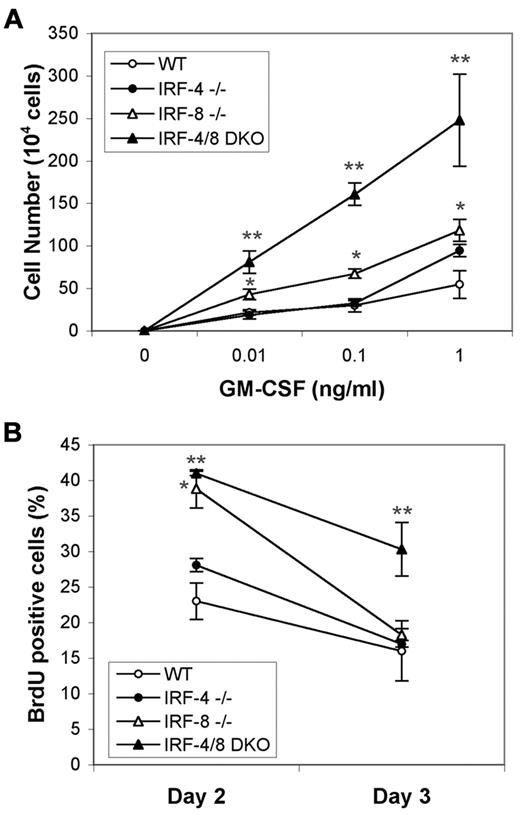
To examine how the loss of IRF-4 and IRF-8 affects growth and differentiation of hematopoietic progenitor cells, lineage-negative (lin−) cells were isolated from BM of wild-type, single KO, and DKO mice and then cultured in the presence of GM-CSF. Quantification of viable cells after 4 days of GM-CSF stimulation shows that, as previously described, IRF-8 KO progenitors are hypersensitive to GM-CSF. However, IRF-4/8 DKO progenitors have a much stronger proliferative response than IRF-8-deficient progenitors (Figure 2A). Interestingly, even though IRF-4 KO mice did not show significant expansion of myeloid cells in vivo, IRF-4 KO progenitors were hypersensitive to the high concentration of GM-CSF, 1 ng/mL (P < .02 compared with wild-type). To further determine whether IRF-4/8 DKO BM progenitors have a greater proliferative capacity, BrdU incorporation assay was performed using 0.1 ng/mL GM-CSF. At 2 days after GM-CSF treatment, increased cell proliferation was seen in both IRF-8 KO and IRF-4/8 DKO progenitors (Figure 2B). However, at 3 days after GM-CSF treatment, whereas proliferation rate of IRF-8 KO progenitors was decreased to the level of wild-type progenitors, IRF-4/8 DKO progenitors showed approximately 2-fold higher cell proliferation than wild-type ones or those with either of the single KO genotypes (Figure 2B). These data indicate that IRF-4/8 DKO progenitors are more sensitive to GM-CSF-stimulated proliferation than single KO progenitors, which probably contributes to the more aggressive CML-like phenotype observed in IRF-4/8 DKO mice. These data suggest a role for IRF-4 in myeloid lineage development as a suppressor of proliferation of myeloid progenitor cells.
IRF-4/8 DKO progenitors are more sensitive to GM-CSF–stimulated proliferation than single KO cells. (A) Lineage-depleted BM cells (3 × 105 cells/well) were cultured for 4 days in the presence of GM-CSF, and viable cells were counted to determine the proliferative response of lin− progenitors to GM-CSF. *P < .035, comparisons between IRF-8 KO and wild-type cells at all 3 concentrations of GM-CSF. **P < .008, comparison between IRF-4/8 DKO and IRF-8 KO cells. (B) Growth of lin− progenitors in the presence of 0.1 ng/mL GM-CSF. BrdU incorporation was monitored. *P < .001, comparison between wild-type and IRF-8 KO cells at day 2. **P < .013 for comparison between wild-type and IRF-4/8 DKO cells at both day 2 and day 3.
IRF-4/8 DKO progenitors are more sensitive to GM-CSF–stimulated proliferation than single KO cells. (A) Lineage-depleted BM cells (3 × 105 cells/well) were cultured for 4 days in the presence of GM-CSF, and viable cells were counted to determine the proliferative response of lin− progenitors to GM-CSF. *P < .035, comparisons between IRF-8 KO and wild-type cells at all 3 concentrations of GM-CSF. **P < .008, comparison between IRF-4/8 DKO and IRF-8 KO cells. (B) Growth of lin− progenitors in the presence of 0.1 ng/mL GM-CSF. BrdU incorporation was monitored. *P < .001, comparison between wild-type and IRF-8 KO cells at day 2. **P < .013 for comparison between wild-type and IRF-4/8 DKO cells at both day 2 and day 3.
IRF-4/8 DKO mice show a greater expansion of granulocytes/macrophage progenitor cells
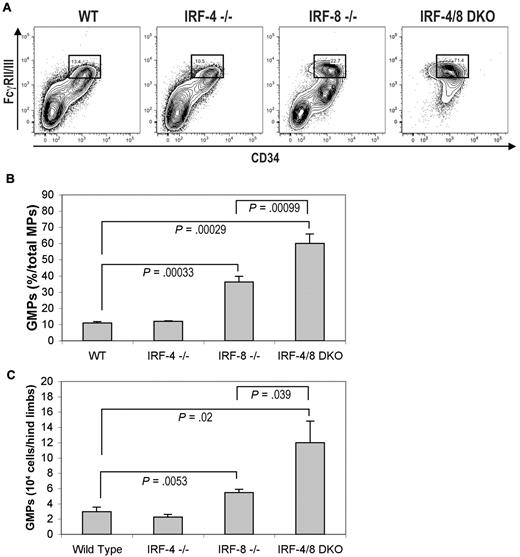
To further examine whether deficiency of IRF-4 and IRF-8 induces aberrations in myeloid development, we analyzed BM myeloid progenitor cells (c-Kit+Sca1−IL-7Rα−lin−) from KO mice. This myeloid progenitor compartment is further separated into common myeloid progenitors (CD34+/FcγRII/IIIlow), megakaryocyte/erythrocyte progenitors (CD34−/FcγRII/III−), and granulocyte/macrophage progenitors (GMPs; CD34+/FcγRII/IIIhigh). IRF-8 KO mice showed a 2-fold increase in GMP fraction compared with wild-type mice, whereas IRF-4 KO mice showed no significant difference from the wild-type ones (Figure 3A-B). A significantly greater increase in GMP fraction was seen in IRF-4/8 DKO mice compared with IRF-8 KO mice (Figure 3A-B). In addition, the absolute number of GMPs in total BM cells from both hind limbs of IRF-8 KO mice was increased approximately 2-fold, whereas that of IRF-4/8 DKO mice was increased approximately 4-fold compared with wild-type controls (Figure 3C).
Flow cytometric analysis of myeloid progenitor cells in wild-type, IRF-4 KO, IRF-8 KO, and IRF-4/8 DKO mice. (A) Numbers in boxed area indicate percent of GMPs in total myeloid progenitor cells. (B) Statistical analysis of the frequency of GMPs in total myeloid progenitor cells. (C) Statistical analysis of the absolute number of GMPs in total BM cells isolated from both hind limbs.
Flow cytometric analysis of myeloid progenitor cells in wild-type, IRF-4 KO, IRF-8 KO, and IRF-4/8 DKO mice. (A) Numbers in boxed area indicate percent of GMPs in total myeloid progenitor cells. (B) Statistical analysis of the frequency of GMPs in total myeloid progenitor cells. (C) Statistical analysis of the absolute number of GMPs in total BM cells isolated from both hind limbs.
We also examined IRF-4 and IRF-8 expression in sorted GMPs. To confirm the GMP sorting, we checked expression pattern of lineage-affiliated genes in purified GMPs as previously described.24 Most myeloid genes tested were expressed in sorted GMPs, except for megakaryocyte/erythrocyte progenitor-affiliated gene β-globin. In contrast, lymphoid genes were not expressed in sorted GMPs (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating that the sorted GMPs were pure. We found that both IRF-4 and IRF-8 are indeed expressed in GMPs (supplemental Figure 1). Although IRF-8 expression has been shown in lin− BM cells,25 our data show, for the first time, that IRF-8 is expressed in the GMP subpopulation. Together, these data suggest that both IRF-4 and IRF-8 function in regulating the proliferation and/or survival of myeloid progenitor cells.
A large number of IRF-4 target genes have also been identified. However, most of these genes were identified in lymphocytes and are related to IRF-4's immune and oncogenic function.26 To gain insights into how loss of IRF-4 affects the transcriptional program underlying leukemogenesis, we analyzed the genome-wide transcription profiles of GMPs from WT, IRF-8 KO, and IRF-4/8 DKO mice (see Supplemental data). Microarray analysis revealed genes differentially expressed in IRF-4/8 DKO and IRF-8 KO GMPs (supplemental Figure 2). Several genes that are down-regulated in both IRF-8 KO and IRF-4/8 DKO GMPs are involved in cell growth regulation and/or tumorigenesis, such as Grb10, Ikzf3 (encoding an Ikarose family member), and Casc4 (cancer susceptibility candidate 4). Some genes are expressed at a higher level in only IRF-8 KO or IRF-4/8 DKO GMPs, suggesting that IRF-4 and IRF-8 play opposite roles in regulating these genes. Expression of Inppl1/SHIP2, which is overexpressed in several cancers,27 is increased in IRF-4/8 DKO GMPs. The significance of genes that are differentially expressed in IRF-4/8 DKO and IRF-8 KO GMPs in leukemogenesis will be studied systematically in the future.
IRF-8 target genes identified in myeloid cell lines that regulate cell growth and survival, such as NF-1,28 c-Myc inhibitors Blimp-1 and Mets,16 tumor suppressor Ink4b,29 as well as antiapoptotic proteins Bcl-217 and Bcl-xL,30 were not found in the genes that are differentially expressed in WT versus IRF-4/8 DKO and IRF-8 KO GMPs. The difference may be the result of different cell types used in the study. At least for NF-1, its expression is not detectable in WT GMPs.31
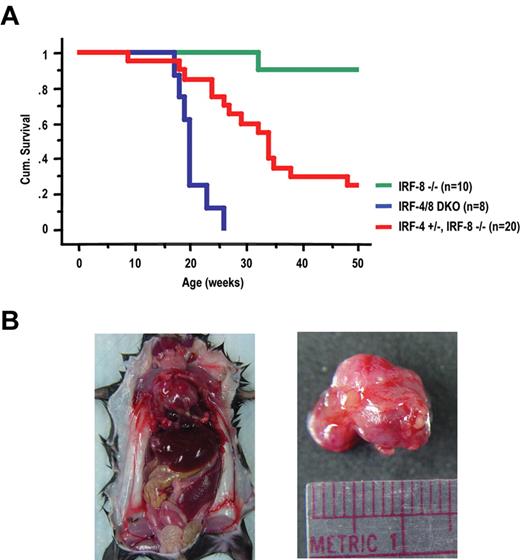
IRF-4/8 DKO mice die of a lymphoblastic leukemia/lymphoma and die much earlier than IRF-8 KO mice
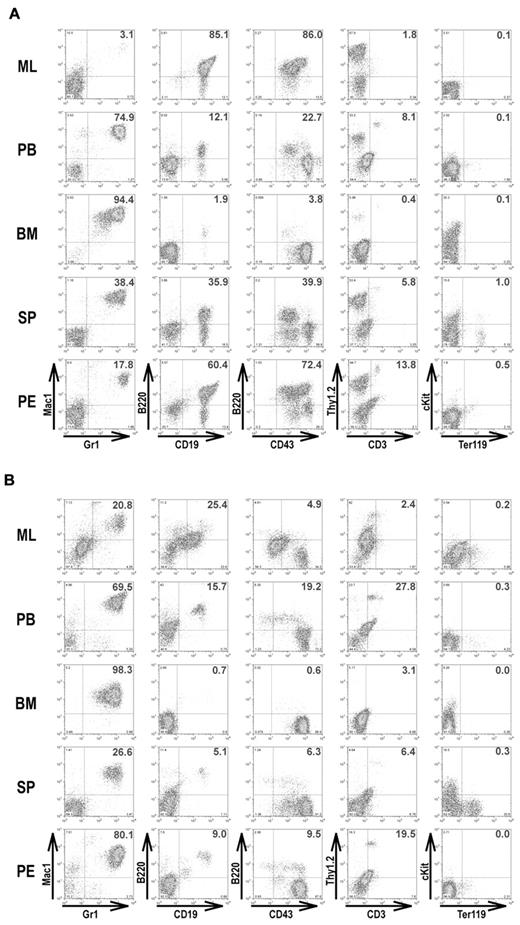
It has been reported that 33% of IRF-8 KO mice became moribund by 50 weeks of age, showing a blast crisis involving both myeloid and lymphoid lineages.11 We compared the survival rate of IRF-4/8 DKO, IRF-8 KO, as well as IRF-4+/−IRF-8−/− mice. All IRF-4/8 DKO mice became moribund or died by 15 to 25 weeks of age (Figure 4A). IRF-4+/−IRF-8−/− mice also became moribund much earlier than IRF-8 KO mice, suggesting a gene dosage effect of IRF-4 in leukemia development. Only one IRF-8 KO mouse died during the time course of this experiment (50 weeks). Gross examination revealed that all IRF-4/8 DKO mice (dead or moribund) developed a massive mediastinal lymphoma (ML), a possible cause of death, as well as hepatosplenomegaly and lymphadenopathy (Figure 4B). Pleural effusion was also seen in most IRF-4/8 DKO mice (Table 1). Flow cytometric analysis showed that the majority of cells in the ML were B-lymphoblasts (B220+, CD19+, CD43+; Figure 5A). Large percentages of B-lymphoblasts were also seen in the pleural effusions. Similar to the early stage of the disease described in “IRF-4/8 DKO mice develop an aggressive CML-like disease much earlier than IRF-8 KO mice,” BM and PB of the diseased mice contain mostly granulocytic cells (Figure 5A; Table 1). Spleens of the diseased mice had a mixture of granulocytic and B-lymphoblastic cells. IRF-4/8 DKO animals therefore develop a lymphoblast leukemia/lymphoma on top of a CML-CP-like MPD.
Cumulative survival of IRF-8 KO, IRF-4/8 DKO, and IRF-4+/−IRF-8−/− mice and gross examination of moribund IRF-4/8 DKO mouse. (A) Survival curves were generated by Kaplan-Meier survival analysis. (B) Gross examination of a representative moribund IRF-4/8 DKO mouse showing massive ML, hepatosplenomegaly, and lymphadenopathy.
Cumulative survival of IRF-8 KO, IRF-4/8 DKO, and IRF-4+/−IRF-8−/− mice and gross examination of moribund IRF-4/8 DKO mouse. (A) Survival curves were generated by Kaplan-Meier survival analysis. (B) Gross examination of a representative moribund IRF-4/8 DKO mouse showing massive ML, hepatosplenomegaly, and lymphadenopathy.
Hematologic analyses of diseased IRF-4/8 DKO and IRF-4+/−/IRF-8−/− mice
| Characteristic/location . | IRF-4/8 DKO (n = 4) . | IRF-4+/−/IRF-8−/− (n = 4) . |
|---|---|---|
| Gr1+Mac1+ (%) | ||
| ML | 2.15 ± 0.73 | 51.1 ± 19.6 |
| PB | 82 ± 7.10 | 78.1 ± 6.53 |
| BM | 95.5 ± 0.97 | 95.6 ± 1.68 |
| SP | 56.7 ± 19.73 | 23.4 ± 9.00 |
| PE | 32.6 ± 17.35 | 40.7 ± 24.83 |
| B220+ (%) | ||
| ML | 69.6 ± 15.06 | 15.9 ± 6.59 |
| PE | 36.45 ± 15.43 | 12.67 ± 4.52 |
| Tissue weight (g) | ||
| SP | 0.38 ± 0.05 | 0.41 ± 0.11 |
| LV | 1.24 ± 0.19 | 0.74 ± 0.15 |
| ML | 0.81 ± 0.14 | 0.08 ± 0.01 |
| Pleural effusion (mL) | 0.49 ± 0.32 | 0.70 ± 0.43 |
| Total BM cells from hind limbs (×107 cells) | 18 ± 3.57 | 9.41 ± 1.99 |
| Characteristic/location . | IRF-4/8 DKO (n = 4) . | IRF-4+/−/IRF-8−/− (n = 4) . |
|---|---|---|
| Gr1+Mac1+ (%) | ||
| ML | 2.15 ± 0.73 | 51.1 ± 19.6 |
| PB | 82 ± 7.10 | 78.1 ± 6.53 |
| BM | 95.5 ± 0.97 | 95.6 ± 1.68 |
| SP | 56.7 ± 19.73 | 23.4 ± 9.00 |
| PE | 32.6 ± 17.35 | 40.7 ± 24.83 |
| B220+ (%) | ||
| ML | 69.6 ± 15.06 | 15.9 ± 6.59 |
| PE | 36.45 ± 15.43 | 12.67 ± 4.52 |
| Tissue weight (g) | ||
| SP | 0.38 ± 0.05 | 0.41 ± 0.11 |
| LV | 1.24 ± 0.19 | 0.74 ± 0.15 |
| ML | 0.81 ± 0.14 | 0.08 ± 0.01 |
| Pleural effusion (mL) | 0.49 ± 0.32 | 0.70 ± 0.43 |
| Total BM cells from hind limbs (×107 cells) | 18 ± 3.57 | 9.41 ± 1.99 |
ML indicates mediastinal lymphoma; PB, peripheral blood; BM, bone marrow; SP, spleen; PE, pleural effusion; and LV, liver.
Immunophenotyping of leukemic cells in moribund IRF-4/8 DKO and IRF-4+/−IRF-8−/− mice. Cells isolated from ML, PB, BM, spleen (SP), and pleural effusion (PE) of moribund IRF-4/8 DKO (A) and IRF-4+/−IRF-8−/− (B) mice were analyzed by flow cytometry. Two-parameter dot plots show expression of lineage-specific antigens as indicated.
Immunophenotyping of leukemic cells in moribund IRF-4/8 DKO and IRF-4+/−IRF-8−/− mice. Cells isolated from ML, PB, BM, spleen (SP), and pleural effusion (PE) of moribund IRF-4/8 DKO (A) and IRF-4+/−IRF-8−/− (B) mice were analyzed by flow cytometry. Two-parameter dot plots show expression of lineage-specific antigens as indicated.
ML was also seen in moribund IRF-4+/−IRF-8−/− mice, but not as dramatically as those of IRF-4/8 DKO animals. Large-volume bloody pleural effusions, the probable cause of death, were seen in all diseased IRF-4+/−IRF-8−/− animals (Table 1). B-lymphoblastic cells were seen in ML, PB, pleural effusions, and spleens of these mice (Figure 5B; Table 1), but most cells in hematopoietic tissues were granulocytes.
Having found that loss of IRF-4 promotes the development of B-lymphoblastic malignancies, we searched the publicly accessible Oncomine database (a cancer microarray database and integrated data-mining platform32 ; https://www.oncomine.org) and found that expression of IRF-4, but not IRF-8, was significantly down-regulated in pediatric B-ALL compared with B-cell precursors from healthy donors (supplemental Figure 3; and data not shown). It has also been shown that IRF-4 expression is decreased in Ph+ B-ALL patients and is restored in response to imatinib treatment.33 These data suggest that down-regulation of IRF-4 is also involved in the pathogenesis of human lymphoblastic leukemia.
Discussion
This study establishes that IRF-4 regulates both lymphopoiesis and myelopoiesis and functions as a tumor suppressor in both lineages and that cooperation between deficiencies of IRF-4 and IRF-8 promotes both myeloid and lymphoid tumorigenesis.
It is interesting that IRF-4/8 DKO mice developed a massive mediastinal B-lymphoblastic lymphoma. This malignant feature is not seen in IRF-8 KO mice. The development of mediastinal B-lymphoblastic lymphoma, therefore, is a result of the cooperation between IRF-4 and IRF-8 deficiencies. Clinically, the majority of lymphoblastic lymphomas are immature T-cell phenotype (70%-80%) and the rest are immature B-cell lineage type. The disease is thought to represent a nodal presentation of the corresponding either B- or T-acute lymphoblastic leukemia (ALL) and is generally treated the same way. However, many areas of uncertainty remain about the pathogenesis of B-lymphoblastic lymphoma, and differences that may exist between it and B-ALL. IRF-4/8 DKO mice provide a valuable new model for studying the pathogenesis of B-lymphoblastic lymphoma.
The finding that loss of IRF-4 promotes the development of B-lymphoblastic leukemia/lymphoma in mice is consistent with the down-regulation of IRF-4 in human B-ALL33 (supplemental Figure 3). In the process of analyzing the tumorigenesis in IRF-4/8 double-deficient mice, we also found that IRF-4 functions as a tumor suppressor in BCR/ABL-induced B-ALL.34 Together, these data indicate that down-regulation of IRF-4 plays an important role in the pathogenesis of lymphoblastic malignancies.
The limited clonal expansion of pre-B cells is driven by both the IL-7 and pre-B cell receptor (pre-BCR) signaling. To gain insights into the mechanism by which IRF-4 affects transcription program underlying pre-B cell development, we have previously performed genome-wide expression analysis of IRF-4/8 DKO pre-B cells after IRF-4 transduction. We found that IRF-4 regulates the gene encoding the chemokine receptor Cxcr4.35 The up-regulation of Cxcr4, the receptor for CXCL12, can promote migration of pre-B cells away from IL-7-expressing stroma cells, an event that is necessary for pre-B cell development. It has also been shown that IRF-4/8 suppress surrogate light chain expression, down-regulate pre-BCR, and inhibit pre-B cell proliferation through Ikarose and Aiolos.36 Together, these studies show that IRF-4 and IRF-8 orchestrate the transition from large, cycling pre-B to small, resting pre-B cells. Clonal expansion of pre-B cells, therefore, would continue without IRF-4/8, predisposing the cells to malignant transformation. These functions of IRF-4/8 may underlie, at least in part, the mechanism by which IRF-4/8 function as tumor suppressors in early B-cell development.
It has been shown that IRF-4 expression is down-regulated in T cells in CML patients18 and that IFN-α treatment reverses down-regulation of IRF-4 and IRF-8 in CML patients.13,18 These data suggest that down-regulation of IRF-4, like that of IRF-8, plays a role in the pathogenesis of CML and that IRF-4 and IRF-8 play important roles in mediating the antitumor activities of IFN-α. It is possible the lack of IRF-4 in T cells contributes to the more aggressive phenotype of CML-like disease in IRF-4/8 DKO mice and to the pathogenesis of human CML. The finding that IRF-4/8 DKO BM progenitors have a greater proliferative capacity than WT or single KO in response to GM-CSF suggests a cell-intrinsic effect as well. The roles of IRF-4 in the pathogenesis of human CML need to be further studied.
The molecular mechanism by which IRF-4 exerts its cell intrinsic, myeloid tumor suppressor function is not clear. IRF-4 and IRF-8 have both redundant and distinct functions.8,20,22,37 In addition, IRF-4 has both nuclear and cytoplasmic functions1,38,39 ; and, as a transcription factor, IRF-4 can function both as a positive regulator and as a repressor.1,26 Systematic analyses of the contributions of various functions of IRF-4 in suppressing myeloid cell expansion are needed to elucidate the mechanism by which IRF-4 functions as a myeloid tumor suppressor.
Our discovery of the tumor-suppressor activity of IRF-4 underscores its functional diversity. IRF-4 has been previously implicated as an oncogene in lymphoid tumors.3-5 Recent studies show that expression of IRF-4 is essential for the maintenance of multiple myeloma cells,40 making IRF-4 an attractive target for the development of therapies for multiple myeloma. The finding of IRF-4 functioning as a tumor suppressor, in particular the haploinsufficiency of IRF-4 in the background of IRF-8 deficiency, raises caution for developing therapies aimed at down-regulating IRF-4. Further studies of the mechanisms by which IRF-4 functions both as an oncoprotein and a tumor suppressor are necessary to develop improved therapies for malignancies involving IRF-4.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Aybike Onur for her assistance with cell culture and analysis of diseased mice and Noreen Francis for her help with cell sorting.
This work was supported by the National Heart, Lung and Blood Institute (grant R01HL083515; R.R.) and the Howard Hughes Medical Institute (J.H.S., H.S.).
National Institutes of Health
Authorship
Contribution: R.R. and H.S. designed research, analyzed data, and wrote the paper; and S.-H.J., J.A., and J.H.S. provided design of the study, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruibao Ren, Rosenstiel Basic Medical Sciences Research Center and Department of Biology, Brandeis University, 415 South St, Mailstop 029, Waltham, MA 02454; e-mail: ren@brandeis.edu; and Harinder Singh, Department of Molecular Genetics and Cell Biology, Howard Hughes Medical Institute, University of Chicago, 929 East 57th St, Chicago, IL 60637; e-mail: hsingh@uchicago.edu.
References
Author notes
S.-H.J., J.H.S., and J.A. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal