Abstract
Thymic stromal lymphopoietins (TSLPs) play critical roles in dendritic cell–mediated immune responses. In this study, we found that human trophoblasts and decidual epithelial cells in maternal-fetal interface of early placentas express TSLP mRNA and protein, but only trophoblast cells secret soluble TSLP. Human decidual CD1c+ DCs (dDCs) highly express the functional TSLP receptor complex TSLP receptor and interleukin-7 receptor-α. Recombinant human TSLP activates CD1C+ decidual DCs and peripheral monocyte-derived DCs with increased costimulatory molecules, major histocompatibility complex class II, and OX-40L. Human TSLP or supernatants from human trophoblasts specifically stimulate dDCs to highly produce interleukin-10 and TH2-attracting chemokine CCL-17. The TSLP-activated dDCs prime decidual CD4+ T cells for TH2 cell differentiation, involved in maternal-fetal immunotolerance. Interestingly, the protein expression of TSLP in normal pregnancy with significant TH2 bias is much higher than that of miscarriage showing TH1 bias at the maternal-fetal interface. Therefore, human trophoblasts may contribute to maternal-fetal tolerance by instructing dDCs to induce regulatory TH2 bias in human early pregnancy via TSLP.
Introduction
The maternal-fetal interface exhibits a TH2 bias characterized by interleukin-4 (IL-4), IL-5, and IL-10 secretion during pregnancy. Failure to establish such an immune milieu or exhibiting a TH1 bias, such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) overexpression, is associated with miscarriage and proposed to cause recurrent spontaneous abortion in humans.1-3
Thymic stromal lymphopoietin (TSLP) is an IL-7-like cytokine, originally cloned from the murine thymic stromal cell line Z210R.1, which supports B-cell development.4 The functional murine TSLP receptor (TSLPR) is a heterodimer consisting of the IL-7 receptor-α (IL-7Rα) chain and a common γ-receptor-like chain.5 Both human TSLP and TSLPR were cloned in 2001 by computational analyses.6 TSLP is produced by human epithelial cells, such as skin keratinocytes, tonsil crypt epithelium, and bronchial epithelial cells, and mediates such allergic inflammation as asthma and atopic dermatitis.7-9 The TSLPR is mainly expressed on dendritic cells (DCs), and TSLP activation of DCs induces a TH2-type inflammatory response through OX40 ligand.10
TSLP treatment causes myeloid DCs to produce large amounts of CCL17 and CCL22, preferentially attracting TH2 cells. In allergic diseases, TSLP-DCs induce robust proliferation of naive allogeneic CD4+ T cells that subsequently differentiate into inflammatory TH2 cells producing high levels of IL-5 and IL-13, moderate IL-4, but little IL-10 and IFN-γ.9 IL-25 enhances the expansion of chemoattractant receptor-homologous molecule (CRTH2) positive TH2 central memory cells induced by TSLP-DCs, and further augments TH2 cytokine production. The enhanced IL-25-induced functions of TH2 memory cells are associated with sustained IL-4–independent expression of GATA-3.11 The peripheral blood monocyte-derived DCs (moDCs) or gut DCs activated by mucosal epithelial cells normally produce a classic TH2 response with high IL-10 and IL-6 levels and low IL-12 levels in human intestine.12 Whether a TSLP-TSLPR signaling pathway is associated with TH2 bias at the human maternal-fetal interface remains unknown.
Here, we showed TSLP protein expression in human trophoblasts and decidual epithelial cells (DECs) at the maternal-fetal interface during early pregnancy and found that decidual DCs (dDCs) expressed the highest level of TSLP receptors among all the decidual cells that were the main target cells of TSLP. Furthermore, we demonstrated that the TSLP-instructed dDCs strongly polarized decidual CD4+ T cells toward classic TH2 bias, which is beneficial for pregnancy.
Methods
Samples
The first-trimester human villous and decidual tissues were obtained from placentas of 15 clinically normal pregnancies (age, 27.30 ± 3.42 years; gestational age at sampling, 8.18 ± 1.45 weeks, mean ± SD), which were terminated for nonmedical reasons, and of 15 miscarriages (age, 28.44 ± 3.45 years; gestational age at sampling, 7.53 ± 1.7 weeks, mean ± SD), which were classified as unexplained after the exclusion of maternal anatomic or hormonal abnormalities, or paternal and maternal chromosomal abnormalities. The endometrial samples were collected from the fertile women (5 cases) during the proliferative or secretory phase of normal menstrual cycle. Each subject completed a signed, written consent form approved by Human Investigation Committee in Hospital of Obstetrics & Gynecology, Fudan University.
Isolation and culture of human trophoblasts
The trophoblast cells were isolated by the trypsin-DNase I digestion and discontinuous Percoll gradient centrifugation, as described by our previous study,13 and cultured in Dulbecco modified Eagle medium (DMEM)–high-glucose complete medium (2mM glutamine, 25mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, 100 IU/mL penicillin, and 100 μg/mL streptomycin) supplemented with 20% heat-inactivated fetal bovine serum (FBS; Invitrogen) in 5% CO2 at 37°C.
Isolation and culture of decidual stromal cells and gland epithelial cells
The decidual tissue (4-6 g) was cut and digested in RPMI 1640 supplemented with collagenase type IV (1.0 mg/mL, CLS-1, Worthington Biomedical) and 1% FBS for 80 minutes at 37°C with gentle agitation. The total suspension was filtrated and enriched by discontinuous Percoll gradient centrifugation. The decidual stromal cells between densities of 1.042 to 1.062 g/mL were recovered, and cultured in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The intact glandular organs retained on the 32-μm wire mesh were back-washed out and cultured in DMEM/Ham F12 for 1 to 2 hours in 5% CO2 at 37°C. The floating glandular organs were transferred to a plate precoated with Matrigel and cultured in complete medium of DMEM/Ham F12 supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Isolation and culture of CD4+ T cells and DCs
The decidual mononuclear cells between densities of 1.062 to 1.077 g/mL were collected, and the decidual CD4+ T cells were isolated by magnetic affinity cell sorting (MACS) using the CD4+ beads kit (Miltenyi Biotec).
The decidual cells between densities of 1.042 to 1.062 g/mL were collected, and decidual DCs were isolated by MACS with the BDCA-1 kit (Miltenyi Biotec). The purity of dDCs fraction was approximately 86.8% plus or minus 7.8% as identified with phycoerythrin (PE)–CD1c by flow cytometry.
The CD14+ monocytes were isolated from the early pregnant peripheral blood mononuclear cells by MACS. The cells passing through the LS columns were enriched for CD4+ T cells by MACS. Purified CD14+ monocytes were cultured with recombinant human IL-4 (rhIL-4; 100 U/mL, PeproTech) and recombinant human granulocyte-macrophage colony-stimulating factor (100 U/mL, PeproTech) for 7 days. The floating cells with short dendrite were further isolated using CD11c bead-based MACS, which were named peripheral blood moDCs with a purity of more than 95%.
The DCs were cultured for 48 hours with culture medium, or medium containing rhTSLP (15 ng/mL), rhIL-7 (50 ng/mL, PeproTech), or lipopolysaccharide (LPS; 10 μg/mL, Sigma-Aldrich). In other experiments, dDCs were cocultured with trophoblasts, with or without neutralizing anti-TSLP antibody (50 μg/mL) in the coculture.
RT-PCR analysis
The mRNA and cDNA templates for TSLP were prepared, and reverse-transcription polymerase chain reaction (RT-PCR) was performed as previously described.13 The primer sequences were as follows: TSLP (131 bp), forward: 5-GCT ATC TGG TGC CCA GGC TAT-3; reverse: 5-CGA CGC CAC AAT CCT TGT AAT-3; housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase; 235 bp), forward: 5-GGG GAG CCA AAA GGG TCA TCA TCT-3; reverse: 5-GAG GGG CCA TCC ACA GTC TTC T-3.
Quantitative RT-PCR
Real-time RT-PCR was performed as our previous methods.14 The primers for human TSLP and GAPDH were the same as in “RT-PCR analysis” above. To compare the T-bet and GATA-3 mRNA in the primed dCD4+ T cells, the following primers were used: GATA-3 (79 bp), forward: 5-GCG GGC TCT ATC ACA AAA TGA-3; reverse: 5-GCT CTC CTG GCT GCA GAC AGC-3; T-bet (75 bp), forward: 5-GAT GTT TGT GGA CGT GGT CTT G-3; reverse: 5-CTT TCC ACA CTG CAC CCA CTT-3.
Immunohistochemistry
The villous and decidual tissue specimens were processed for immunohistochemical staining, and sheep anti–human TSLP, IFN-γ, TNF-α, IL-4, or IL-10 primary antibody (R&D Systems) was used as primary antibodies, incubated overnight at 4°C, followed by incubation with a biotinylated rabbit anti–sheep (KPL) secondary antibody, then by the avidin-biotin–horseradish peroxidase complex, and were stained with diaminobenzidene and counterstained with hematoxylin. The immunocytochemistry experiments were performed with the same antibodies on the correspondent cells. All images were captured by Olympus BX51 microscopy. Original magnification was ×200 for all panels. Olympus Micro DP70 and DP controller software were used to acquire images digitally. Each image was saved in TIF form, and all images were manipulated with Photoshop 7.0.1.
Coculture of DCs and CD4+ T cells
The dDCs were cultured with culture medium, or medium containing rhTSLP (15 ng/mL), LPS (10 μg/mL, Sigma-Aldrich), or directly contacted with the primary trophoblast cells. After 24 hours of culture, CD11c+ DCs were collected and washed 3 times with phosphate-buffered saline to remove excess cytokines. The remaining cells were cocultured with autologous or allogenetic decidual CD4+ T cells with different DC/T-cell ratios. The decidual CD4+ T cells were activated with 5 μg/mL anti-CD3, 1 μg/mL anti-CD28, and 20 U/mL rhIL-2 for 3 days, and collected, washed, then incubated with culture medium only, or medium containing various concentrations of rhTSLP (0.05, 0.5, 5.0, and 50.0 ng/mL). After 5 days of coculture, the decidual CD4+ T cells were reactivated with anti-CD3 (OKT3) and anti-CD28 (28.2; eBioscience) for 24 hours before the supernatants were collected.
Western blot for TSLP and its receptors
A total of 50 μg of protein from the different cells was subjected to 10% or 15% sodium dodecyl sulfate-polyacrylamide gel for TSLPR, IL-7Rα, TSLP, and GAPDH, respectively. The proteins were transferred to polyvinylidene difluoride membranes, which were blocked and immunoblotted with goat anti-TSLPR (1:1000, R&D Systems), mouse anti-IL-7Ra (1:1000, Abcam), sheep anti-TSLP (1:1000, R&D Systems), and rabbit anti-GAPDH (1:5000, KangChen) overnight at 4°C. The bounded antibodies were visualized using peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) followed by detection using an ECL Kit (Pierce Chemical) on Las-300 (FujiFilm).
ELISA
The supernatant from each indicated group was collected and centrifuged at 2000g and stored at −80°C. The amount of human cytokines, including IL-4, IL-5, IL-10, TNF-α, and IFN-γ, in the culture supernatants was quantified using the cytokine-specific enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer's instructions.
Flow cytometry
The freshly isolated decidual mononuclear cells were stained with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (mAb) against human CD1c, CD3, CD14, CD19, CD56; PE-conjugated anti–human TSLPR, IL-7Rα, or their corresponding isotype controls (eBioscience). The purified decidual and peripheral blood CD4+ T cells were stained with FITC-conjugated mAb against human CD4 or CCR7, PE-conjugated anti–human CD45RA or CRTH2, PE/Cy5-conjugated anti–human CD45RO, and isotype control (eBioscience).
For intracellular cytokine production by quadruple labeling, CD4+ CD45RO+CCR7+ T cells were gated and the expression of TNF-α or IL-10 was analyzed using FITC-conjugated anti-CD4 and PE/Cy5-conjugated anti-CD45RO, allophycocyanin-conjugated anti-CCR7, and PE-conjugated anti–TNF-α or IL-10 mAbs. For triplicate labeling, CD4+CRTH2+ T cells were gated and analyzed for the expression of TNF-α or IL-10 with FITC-conjugated anti-CD4 and PE/Cy5-conjugated anti-CRTH2 and PE-conjugated anti–TNF-α or IL-10 mAbs, and then analyzed in a FACSCalibur flow cytometer (BD Biosciences) using CellQuest 3.3 software.
Statistical analysis
Statistical analysis was performed using the SPSS statistics software package (SPSS 11.5). Statistical comparisons for the TSLP expression or cytokines in the supernatant used 2-tailed Student t test or one-way analysis of variance. The post hoc Dunnett t test was used to compare the significance levels between control and various treatments. All error bars in the figures indicate SD. Statistical significance was accepted at P values less than .05.
Results
TSLP is expressed at human maternal-fetal interface in early pregnancy
We first screened samples by RT-PCR for TSLP mRNA expression in villi and decidua from women in early pregnancy. As shown in Figure 1A, TSLP mRNA is detected in all these tissues, as well as secretory-phase endometrium. The primary trophoblasts, DECs, and stromal cells also express TSLP mRNA. TSLP mRNA levels in villi and decidua are significantly higher than that in secretory endometrium by semiquantitative RNA analysis (P = .001 and P = .041, respectively), and trophoblasts express significantly more TSLP mRNA than decidual stromal cells (P < .002). The results are highly reproducible in 4 independent experiments.
TSLP is expressed at human maternal-fetal interface in the early pregnancy. TSLP expression was evaluated by RT-PCR (A), immunohistochemistry (B), and immocytochemistry (C). Both villi and decidua from the early pregnancy and endometrial tissues from nonpregnant women expressed TSLP mRNA. The primary human trophoblasts (Tros), DECs, and DSCs also expressed TSLP mRNA (A). TSLP protein was localized in the cytoplasm of Tros (single arrow, cytotrophoblast cells; double arrow, syncytiotrophoblast cells) in villi and the cytoplasm of DECs (↑) in decidua (B), but absent in DSCs (▴). TSLP protein could not be detected in endometrium of the nonpregnancy, including proliferative and secretory phase (B). These results were further confirmed in the primary trophoblasts, DECs, and DSCs (C). Data are mean ± SD. The experiments were repeated 4 times with 4 placenta, decidua and endometrial tissue samples, respectively. The picture is a representative one.
TSLP is expressed at human maternal-fetal interface in the early pregnancy. TSLP expression was evaluated by RT-PCR (A), immunohistochemistry (B), and immocytochemistry (C). Both villi and decidua from the early pregnancy and endometrial tissues from nonpregnant women expressed TSLP mRNA. The primary human trophoblasts (Tros), DECs, and DSCs also expressed TSLP mRNA (A). TSLP protein was localized in the cytoplasm of Tros (single arrow, cytotrophoblast cells; double arrow, syncytiotrophoblast cells) in villi and the cytoplasm of DECs (↑) in decidua (B), but absent in DSCs (▴). TSLP protein could not be detected in endometrium of the nonpregnancy, including proliferative and secretory phase (B). These results were further confirmed in the primary trophoblasts, DECs, and DSCs (C). Data are mean ± SD. The experiments were repeated 4 times with 4 placenta, decidua and endometrial tissue samples, respectively. The picture is a representative one.
The villous and decidual tissues from early pregnancy are positively stained for TSLP by immunohistochemistry, and syncytiotrophoblasts and cytotrophoblasts in villi and invasive trophoblast cells in decidua expressed TSLP protein (Figure 1B). However, the nonpregnant endometrium in either proliferative or secretory phase does not (Figure 1B). DECs are also positive for TSLP protein, whereas the large, pale stromal cells are TSLP-negative. These results were further confirmed by immunocytochemistry that both primary trophoblasts and DECs express TSLP protein in cytoplasm, but decidual stromal cells do not (Figure 1C).
The primary trophoblasts continuously secrete soluble TSLP into culture supernatant from 12 to 96 hours, accumulating to 19.59 plus or minus 1.12 pg/mL in 48 hours of culture from cells at a density of 1 × 106/mL. However, TSLP is undetectable in supernatants of either the primary decidual epithelial or stromal cells (data not shown).
TSLP functional receptors, TSLPR and IL-7Rα, are expressed on decidual lymphocyte cells
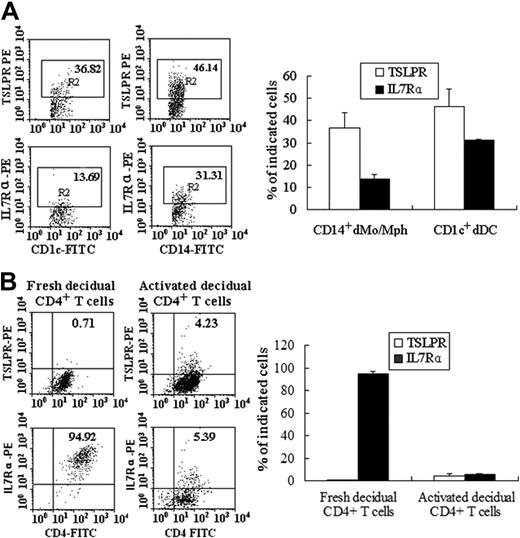
The cellular targets of TSLP have been identified to be lymphocytes and granulocytes. So we next detected the expression of TSLPR and IL-7Rα, the TSLP functional receptor on decidual mononuclear cells from early pregnancy by flow cytometry. Decidual CD14+ monocyte/macrophage (Mo/Mph), CD1c+ DCs, are the main cells expressing surface TSLPR, whereas CD3+ T cells and CD56bright uterine NK cells express little TSLPR (data not shown). The prevalence of TSLPR on the peripheral Mo/Mph and DCs in the freshly isolated decidual mononuclear cells is 25.34% plus or minus 5.94% and 41.62% plus or minus 6.84%, respectively (Figure 2A). IL-7Rα is also observed to be expressed on peripheral CD14+ Mo/Mph and CD1c+ DCs, with 13.69% plus or minus 1.79% of peripheral CD14+ Mo/Mph and 31.5% plus or minus 0.318% of decidual CD1c+ DCs express IL-7Rα, respectively (Figure 2A). In contrast to little TSLPR expression, 94.92% plus or minus 2.33% of CD4+ T cells are IL-7Rα–positive cells (Figure 2B). It is more surprising that activation with anti-CD3 and anti-CD28 antibodies significantly increases TSLPR expression level but remarkably decreases IL-7Rα expression on CD4+ T cells (Figure 2B).
TSLP receptor is expressed on decidual CD14+ Mo/Mph, CD1c+ DCs, and activated dCD4+ T cells. Flow cytometry was used to analyze the expression of TSLP receptor (TSLPR and IL-7Rα) on decidual CD14+ Mo/Mph, CD1c+ DCs. Both TSLPR and IL-7Rα are expressed on decidual CD14+ Mo/Mph, CD1c+ DCs (A). Stimulation with anti-CD3 and anti-CD28 antibodies significantly increased TSLPR expression level but decreased IL-7Rα expression on CD4+ T cells (B). Bar represents the mean ± SD of 4 repeated experiments from 4 cases.
TSLP receptor is expressed on decidual CD14+ Mo/Mph, CD1c+ DCs, and activated dCD4+ T cells. Flow cytometry was used to analyze the expression of TSLP receptor (TSLPR and IL-7Rα) on decidual CD14+ Mo/Mph, CD1c+ DCs. Both TSLPR and IL-7Rα are expressed on decidual CD14+ Mo/Mph, CD1c+ DCs (A). Stimulation with anti-CD3 and anti-CD28 antibodies significantly increased TSLPR expression level but decreased IL-7Rα expression on CD4+ T cells (B). Bar represents the mean ± SD of 4 repeated experiments from 4 cases.
TSLP up-regulates the expression of surface molecules of human peripheral moDCs and decidual DCs
As shown in Figure 3, rhTSLP alone differently up-regulates expression of cell surface molecules, CD40, CD83, OX-40L, CD80, CD86, and HLA-DR on the decidual DCs compared with the control. There is no difference between TSLP treatment and LPS treatment, a potent DC activator. Similar results are obtained in the peripheral moDCs stimulated by rhTSLP or LPS.
The rhTSLP promotes DC maturation. Decidual DCs and peripheral blood moDCs were stimulated with TSLP or LPS, or cultured in medium only. CD40, CD83, OX-40L, CD80, CD86, and HLA-DR expression was determined by flow cytometry. Filled histograms represent staining of the cells with the markers on the top of histograms; and open histograms, the isotype control (A). The numbers in the histograms indicate the percentage of positive-staining cells in DCs (B).TSLP up-regulates CD40, CD83, OX-40L, CD80, CD86, and HLA-DR expression on DCs compared with the control. The phenotypic changes on moDCs were similar to dDCs. Numbers indicate the percentage of positive-staining cells on DCs (B). The results shown are from 4 independent experiments with 4 cases. Data are mean ± SD. The picture is a representative one.
The rhTSLP promotes DC maturation. Decidual DCs and peripheral blood moDCs were stimulated with TSLP or LPS, or cultured in medium only. CD40, CD83, OX-40L, CD80, CD86, and HLA-DR expression was determined by flow cytometry. Filled histograms represent staining of the cells with the markers on the top of histograms; and open histograms, the isotype control (A). The numbers in the histograms indicate the percentage of positive-staining cells in DCs (B).TSLP up-regulates CD40, CD83, OX-40L, CD80, CD86, and HLA-DR expression on DCs compared with the control. The phenotypic changes on moDCs were similar to dDCs. Numbers indicate the percentage of positive-staining cells on DCs (B). The results shown are from 4 independent experiments with 4 cases. Data are mean ± SD. The picture is a representative one.
Trophoblast cells instruct decidual DCs to secrete high levels of TH2 cell–attractive CCL17 and IL-10 via TSLP secretion
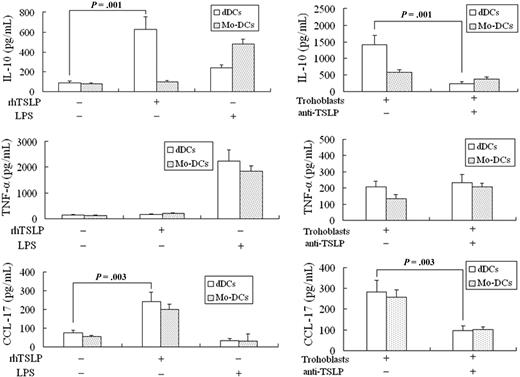
We then investigated whether TSLP could activate decidual DCs to produce high levels of TH2 cell-attractive CCL17 and IL-10 because it can increase the expression of costimulatory molecules, major histocompatibility complex class II, and OX-40L on peripheral moDCs and decidual DCs. The CD1c+ decidual DCs and peripheral blood moDCs were treated with rhTSLP (15 ng/mL) or LPS (10 μg/mL), respectively, for 48 hours. The results show that rhTSLP strongly stimulates decidual DCs to secrete CCL17 and IL-10 as measured by ELISA (Figure 4A), but not TNF-α. IL-10 secreted by peripheral moDCs from early pregnancy is significantly lower than that of decidual DCs, indicating the functional difference between these cells.
rhTSLP and trophoblast cells induce decidual DCs to produce high levels of IL-10 and CCL17. The peripheral monocytes from normal early pregnant women were cultured with rhIL-4 (100 U/mL) and granulocyte-macrophage colony-stimulating factor (100 U/mL) for 5 to 9 days. At day 7, the cells became star-like morphologically. The CD1c+ dDCs or peripheral moDCs from the early pregnancy were treated with rhTSLP (15 ng/mL) or LPS (10 μg/mL) respectively, for 48 hours. The rhTSLP significantly stimulates dDCs to release higher levels of TH2 cell-attractive CCL17 and IL-10. TNF-α production by dDCs remains at a lower level, except for LPS-dDCs, whereas the rhTSLP-treated moDCs secrete lower levels of TNF-α and IL-10. The dCD1c+ DCs or moDCs from the early pregnancy were cocultured with trophoblast cells, with or without 10 μg/mL antihuman TSLP neutralizing antibody (trophoblasts + α-TSLP) for 48 hours. Human trophoblast cells stimulate the decidual DCs to secrete high levels of IL-10 and CCL-17, which can be effectively inhibited by neutralizing anti-TSLP antibody. The amount of TNF-α secreted by the trophoblast-regulated moDCs is not significantly different from that of the trophoblast-regulated dDCs, and α-TSLP has no significant effect on them. Data are from 4 independently conducted experiments with 4 cases. Data are mean ± SD.
rhTSLP and trophoblast cells induce decidual DCs to produce high levels of IL-10 and CCL17. The peripheral monocytes from normal early pregnant women were cultured with rhIL-4 (100 U/mL) and granulocyte-macrophage colony-stimulating factor (100 U/mL) for 5 to 9 days. At day 7, the cells became star-like morphologically. The CD1c+ dDCs or peripheral moDCs from the early pregnancy were treated with rhTSLP (15 ng/mL) or LPS (10 μg/mL) respectively, for 48 hours. The rhTSLP significantly stimulates dDCs to release higher levels of TH2 cell-attractive CCL17 and IL-10. TNF-α production by dDCs remains at a lower level, except for LPS-dDCs, whereas the rhTSLP-treated moDCs secrete lower levels of TNF-α and IL-10. The dCD1c+ DCs or moDCs from the early pregnancy were cocultured with trophoblast cells, with or without 10 μg/mL antihuman TSLP neutralizing antibody (trophoblasts + α-TSLP) for 48 hours. Human trophoblast cells stimulate the decidual DCs to secrete high levels of IL-10 and CCL-17, which can be effectively inhibited by neutralizing anti-TSLP antibody. The amount of TNF-α secreted by the trophoblast-regulated moDCs is not significantly different from that of the trophoblast-regulated dDCs, and α-TSLP has no significant effect on them. Data are from 4 independently conducted experiments with 4 cases. Data are mean ± SD.
TSLPR is lowly expressed on the freshly isolated decidual CD4+ T cells but significantly increases on activation by anti-CD3 and anti-CD28. However, TSLP does not directly affect TH polarization because the ratio of IL-4/IFN-γ in decidual CD4+ T-cell supernatants does not alter significantly after rhTSLP treatment (data not shown). These results imply that TSLP may play a role in the decidual TH2-cell differentiation by instructing decidual DCs.
To further determine whether trophoblasts instruct decidual DCs to secrete high levels of TH2 cell–attractive CCL17 and IL-10 via TSLP secretion, decidual DCs or peripheral moDCs were cocultured with human trophoblasts with or without neutralizing anti-hTSLP antibody (10 μg/mL) for 48 hours. The trophoblasts strongly stimulate decidual DCs to secrete TH2 cell-attractive chemokine CCL17 and IL-10 as detected by ELISA, but not TNF-α (Figure 4B). The anti-TSLP neutralizing antibody significantly inhibits the trophoblast-induced secretion of IL-10 and CCL17, implying a TSLP-dependent process. Although trophoblasts increase IL-10 secretion by peripheral moDCs in early pregnancy, the level is significantly lower than that of decidual DCs. The peripheral moDCs also produce significant amounts of CCL17 in coculture with trophoblasts without neutralizing TSLP antibody (Figure 4B).
TSLP-instructed decidual DCs polarize decidual CD4+ T cells toward TH2 bias
We next investigated whether the TSLP-instructed dDCs (TSLP-dDCs) could polarize decidual CD4+ T cells toward a TH2 bias. The dDCs or moDCs, pretreated for 48 hours with rhTSLP (15 ng/mL), rhIL-7 (50 ng/mL), or LPS (10 μg/mL), were cocultured with allogenetic decidual or peripheral CD4+ T cells for 5 days, respectively, and then IL-4, IL-5, IL-10, IFN-γ, and TNF-α in the supernatant were determined by ELISA. The rhTSLP-dDCs markedly up-regulate IL-4 and IL-10, but only slightly up-regulate IFN-γ and TNF-α from decidual CD4+ T cells, compared with decidual CD4+ T cells cocultured with dDCs but without TSLP (Figure 5). However, no such trend is found for rhIL-7 or LPS-activated dDCs, suggesting that rhTSLP-dDCs can polarize decidual CD4+ T cells toward a TH2-biased profile.
TSLP-treated DCs induce CD4+ T cells to polarize toward a TH2 bias. The 2 kinds of DCs were exposed, respectively, to 15 ng/mL rhTSLP (TSLP-DCs), 50 ng/mL rhIL-7 (IL-7-DCs), or 10 μg/mL LPS (LPS-DCs) for 24 hours, washed 3 times, and then cocultured, respectively, with decidual CD4+ T (dCD4+ T) or peripheral CD4+ T (pCD4+ T) cells for 5 days. Thereafter, the T cells were transferred to a new 96-well round-bottom plate precoated with anti-CD3 and anti-CD28 and cultured for 24 hours. The cytokine secretion of the CD4+ T cells was determined by ELISA. IL-4, IL-5, and IL-10 secretions of the decidual CD4+ T cells cocultured with the TSLP-treated decidual DCs are highly up-regulated, but no such trend is observed for IFN-γ and TNF-α production, compared with the primary DCs. The moDCs pretreated by rhTSLP induce peripheral CD4+ T cells (pCD4+ T) to produce higher levels of IL-4, IL-5, and TNF-α, but lower levels of IL-10 and IFN-γ. Data are the means ± SD from 4 independently conducted experiments with 4 samples.
TSLP-treated DCs induce CD4+ T cells to polarize toward a TH2 bias. The 2 kinds of DCs were exposed, respectively, to 15 ng/mL rhTSLP (TSLP-DCs), 50 ng/mL rhIL-7 (IL-7-DCs), or 10 μg/mL LPS (LPS-DCs) for 24 hours, washed 3 times, and then cocultured, respectively, with decidual CD4+ T (dCD4+ T) or peripheral CD4+ T (pCD4+ T) cells for 5 days. Thereafter, the T cells were transferred to a new 96-well round-bottom plate precoated with anti-CD3 and anti-CD28 and cultured for 24 hours. The cytokine secretion of the CD4+ T cells was determined by ELISA. IL-4, IL-5, and IL-10 secretions of the decidual CD4+ T cells cocultured with the TSLP-treated decidual DCs are highly up-regulated, but no such trend is observed for IFN-γ and TNF-α production, compared with the primary DCs. The moDCs pretreated by rhTSLP induce peripheral CD4+ T cells (pCD4+ T) to produce higher levels of IL-4, IL-5, and TNF-α, but lower levels of IL-10 and IFN-γ. Data are the means ± SD from 4 independently conducted experiments with 4 samples.
The peripheral moDCs from early pregnancy pretreated by rhTSLP cause the peripheral CD4+ T cells to produce high levels of IL-4, IL-5, and TNF-α but low levels of IL-10 and IFN-γ, consistent with other reports.8,9 These results indicate that decidual DCs and peripheral moDCs play different roles in the TH1/TH2 balance at the maternal-fetal interface.
TH1 and TH2 cell differentiation is regulated by key transcriptional factors, such as T-bet for TH1 and GATA-3 for TH2 cells.15 TH1 cells express high levels of T-bet but low levels of GATA-3. We therefore examined the kinetics of GATA-3 and T-bet transcription by quantitative real-time RT-PCR in the decidual CD4+ T cells primed by the primary, TSLP-instructed, or LPS-stimulated dDCs. Although decidual CD4+ T cells cocultured with the primary dDCs express low levels of GATA-3 and T-bet at any time point, the allogeneic decidual CD4+ T cells primed by the TSLP-dDCs express high levels of GATA-3 but low T-bet (data not shown). By contrast, the LPS-DCs induce decidual CD4+ T cells to express high levels of T-bet and low GATA-3.
Decidual and peripheral blood CD4+ T cells have different surface phenotypes
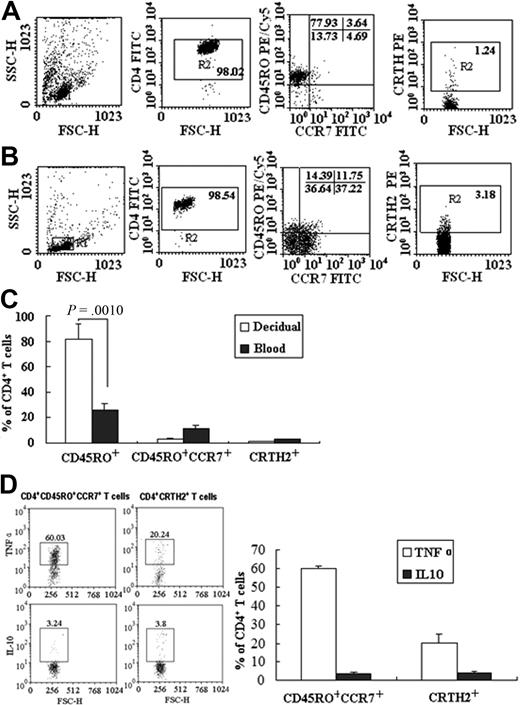
Human CD4+ memory T cells are defined as CD45RO+CCR7+ central memory cells (Tcm) and CD45RO+CCR7− effector memory cells (Tem) by their distinctive surface phenotype, homing capacity, and effector functions.16 Tcm can undergo homeostatic proliferation and differentiate into effectors, and Tem with a reduced proliferative capacity can elicit immediate effector functions. Tcm and Tem can be further divided into subsets producing TH1 or TH2 cytokines with differential chemokine and tissue-homing receptor expressions, linking memory T-cell subsets with polarized TH1 or TH2 function.17 CD4+CRTH2+ T cells produce IL-4, IL-5, and IL-13 but not IFN-γ on T-cell receptor triggering and represent TH2 central memory cells. Because the positively isolated total CD4+ T cells in this study produce different TH cytokines, especially IL-10 and TNF-α, we thus examined the CD4+ T-cell phenotypes to understand this difference. The decidual CD4+ T cells consisted of 77.57% plus or minus 11.92% of CD45RO+ memory T cells, Tcm constitute 3.64% plus or minus 0.62% of total decidual CD4+ T cells, whereas the decidual CD4+CRTH2+ T cells are only 1.24% plus or minus 0.13% (Figure 6A,C). However, the minority of peripheral blood CD4+ T cells is CD45RO+ memory T cells, which occupy 25.14% plus or minus 4.22%; the Tcm are 10.75% plus or minus 4.35%, and the peripheral CD4+ CRTH2+ T cells are 3.18% plus or minus 2.42% (Figure 6B-C). These results suggest that 2 groups of CD4+ T cells exist in human decidua. Subsequently, we investigated which population produces IL-10 and TNF-α on TSLP stimulation. The results in Figure 6D show that 60.03% plus or minus 1.21% of TSLP-treated CD4+CD45RO+CCR7+ T cells express TNF-α and 3.24% plus or minus 1.31% of these cells express IL-10. In contrast, 20.24% plus or minus 4.20% of CD4+CRTH2+ T cells produce TNF-α and 3.8% plus or minus 0.93% of these cells express IL-10. These data have demonstrated that TSLP treatment induces IL-10 production in both groups of CD4+ T cells but induces TNF-α production mainly by Tcm. These results indicate that alteration of IL-10 and TNF-α production may be the result of functional differences between dDCs and peripheral moDCs, and relatively less Tcm and more CD4+CRTH2+ T cells at the maternal-fetal interface contribute to the generation of Th2 bias.
Decidual and peripheral CD4+T cells show different surface phenotypes and function. Flow cytometry was used to investigate the surface phenotype and cytokine production of the decidual and peripheral CD4+ T cells (A-D). The decidual CD4+ T cells constitute 77.57% ± 11.92% of CD45RO+ memory T cells, 3.64% ± 0.62% of CD45RO+CCR7+CD4+ T cells, and 1.24% ± 0.13% of dCD4+CRTH2+ T cells (A,C). The CD45RO+ memory T cells include approximately 25.14% ± 4.22% of the pCD4+ T cells, the CCR7+CD45RO+ Tcm are 10.75% ± 4.35%, and pCD4+CRTH2+ T cells are approximately 3.18% ± 2.42% (B-C). Cytokine production by decidual CD45RO+CCR7+CD4+ T cells and CD4+CRTH2+ T cells was determined by flow cytometry (D). Data are the means ± SD from 4 independently conducted experiments with 4 samples, and compared in the histogram (C-D).
Decidual and peripheral CD4+T cells show different surface phenotypes and function. Flow cytometry was used to investigate the surface phenotype and cytokine production of the decidual and peripheral CD4+ T cells (A-D). The decidual CD4+ T cells constitute 77.57% ± 11.92% of CD45RO+ memory T cells, 3.64% ± 0.62% of CD45RO+CCR7+CD4+ T cells, and 1.24% ± 0.13% of dCD4+CRTH2+ T cells (A,C). The CD45RO+ memory T cells include approximately 25.14% ± 4.22% of the pCD4+ T cells, the CCR7+CD45RO+ Tcm are 10.75% ± 4.35%, and pCD4+CRTH2+ T cells are approximately 3.18% ± 2.42% (B-C). Cytokine production by decidual CD45RO+CCR7+CD4+ T cells and CD4+CRTH2+ T cells was determined by flow cytometry (D). Data are the means ± SD from 4 independently conducted experiments with 4 samples, and compared in the histogram (C-D).
Lower TSLP and its receptor expression and TH 2 bias at the maternal-fetal interface from miscarriage
Because successful pregnancy is characterized by TH2 bias at maternal-fetal interface and failure to establish such an immune milieu or exhibiting a TH1 bias is associated with miscarriage, we then investigated whether there is some difference on the expression of TSLP and its receptors at the maternal-fetal interface between the normal pregnancy and miscarriage.
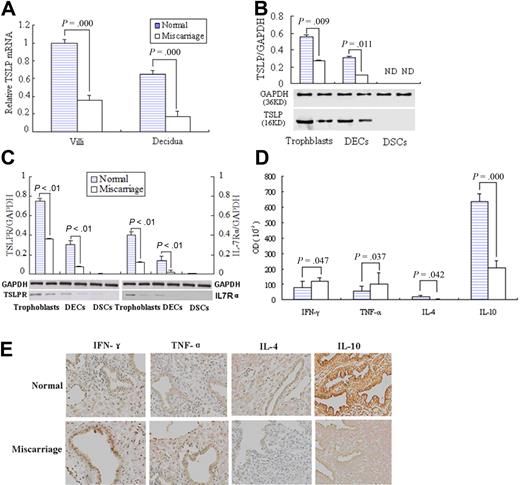
We first analyzed the TSLP mRNA level in the placenta by quantitative RT-PCR. The villous tissues from early normal pregnancy present significantly higher TSLP mRNA level than that of the unexplained miscarriage (P < .01). The same result is obtained in the decidua (Figure 7A).
Higher TSLP and its receptor expression and TH2 bias at maternal-fetal interface in the early pregnancy than miscarriage. Quantitative PCR shows that TSLP expression in villi and decidua from placenta of normal early pregnancy was significantly higher than that of unexplained miscarriage (A). Western blot analysis of TSLP, TSLPR, and IL-7Rα in freshly isolated trophoblast cells, DSCs, DECs, and DSCs from normal early pregnancy and unexplained miscarriage was performed (B-C). The bands were scanned and analyzed on Las-300 (FujiFilm), and the ratio of TSLP, TSLPR, or IL-7Ra to GAPDH indicates the relative protein levels in each group, respectively. The TH1 cytokines, IFN-γ and TNF-α, or TH2 cytokines, IL-4 and IL-10, at the maternal-fetal interface were evaluated by immunohistochemistry (D). Histogram shows the relative expression level of the indicated cytokines as determined by densitometric analysis (E). Data are mean ± SD from 4 repeated experiments with 4 placenta samples, respectively. Original magnification in the representative results is 200-fold (E).
Higher TSLP and its receptor expression and TH2 bias at maternal-fetal interface in the early pregnancy than miscarriage. Quantitative PCR shows that TSLP expression in villi and decidua from placenta of normal early pregnancy was significantly higher than that of unexplained miscarriage (A). Western blot analysis of TSLP, TSLPR, and IL-7Rα in freshly isolated trophoblast cells, DSCs, DECs, and DSCs from normal early pregnancy and unexplained miscarriage was performed (B-C). The bands were scanned and analyzed on Las-300 (FujiFilm), and the ratio of TSLP, TSLPR, or IL-7Ra to GAPDH indicates the relative protein levels in each group, respectively. The TH1 cytokines, IFN-γ and TNF-α, or TH2 cytokines, IL-4 and IL-10, at the maternal-fetal interface were evaluated by immunohistochemistry (D). Histogram shows the relative expression level of the indicated cytokines as determined by densitometric analysis (E). Data are mean ± SD from 4 repeated experiments with 4 placenta samples, respectively. Original magnification in the representative results is 200-fold (E).
TSLP protein expression in trophoblasts, decidual stromal cells, and epithelial cells from the normal early pregnancy or unexplained miscarriage was also evaluated by Western blot (Figure 7B). The ratio of hTSLP/GAPDH in trophoblasts from the normal early pregnancy is 0.58 plus or minus 0.02, significantly higher than that of the miscarriage, 0.32 plus or minus 0.02. The ratio of DECs from normal pregnancy was 0.29 plus or minus 0.01, also significantly higher than the 0.14 plus or minus 0.01 of miscarriage.
We also examined the expression of TSLP receptors, TSLPR and IL-7Rα, in these cells from normal pregnancy and miscarriage. As shown in Figure 7C, the ratio of TSLPR/GAPDH in trophoblasts and DECs from normal pregnancy is significantly higher than that of the miscarriage. The similar change is observed on IL-7Rα expression. There is no expression of TSLP and its receptors TSLPR/ IL-7Rα protein in decidual stromal cells (DSCs) either from normal pregnancy or miscarriage.
Meanwhile, the TH1 cytokines, IFN-γ and TNF-α, or TH2 cytokines, IL-4 and IL-10, at the maternal-fetal interface were also evaluated by immunohistochemistry and show a significant TH2 bias in the early pregnancy compared with the TH1 bias in the miscarriage (Figure 7D-E).
Discussion
The human maternal-fetal interface is characterized by intimate contact between the maternal tissue and the extravillous trophoblast cells that invade the decidua. High amounts of different leukocytes are present within the stromal compartment of the luteal phase endometrium, which increase in the first trimester decidua.18 The most abundant leukocytes are CD56brightCD16− NK cells, CD38+CD2+/−CD3−CD16−CD68+ macrophages, and CD3+ T cells (including both CD4+ and CD8+); although B cells are virtually absent,19 DCs are also present within the decidua.20 These various cells interact and play critical roles in modulation of placentation and fetal development.21
We have found that only trophoblasts secret soluble TSLP at the maternal-fetal interface. Both TSLP and its functional receptors TSLPR and IL-7Rα are expressed on human trophoblasts and DECs, but not in DSCs, and the TSLP and its receptors expression in the unexplained miscarriage are significantly decreased, suggesting that TSLP plays a role in human normal early pregnancy.
The resident DCs in the decidua are in close contact with extravillous trophoblasts usually at the decidua basalis.22 Moreover, the dDCs are the main cells expressing both TSLPR and IL-7Rα, forming functional TSLP receptors. TSLP secreted by trophoblasts is a DC instructor different from other DC activation factors, such as LPS. TSLP induces decidual DCs to secrete high levels of IL-10 and TH2 cell-attractive chemokine CCL17, but not the proinflammatory cytokine TNF-α. By secreting TSLP, the trophoblasts endow decidual DCs with the ability to prime total decidual CD4+ T cells to produce higher IL-5, IL-4, and IL-10 levels and minimal inflammatory cytokines, such as TNF-α and IFN-γ. However, rhTSLP does not induce peripheral moDCs from early pregnancy to produce IL-10 and TNF-α. The rhTSLP-DCs also prime peripheral naive CD4+ T cells to produce high levels of TNF-α and IL-5 and moderate IL-4, but not IL-10 or IFN-γ.9 These findings suggest that TSLP at the maternal-fetal interface may play a critical role in maternal-fetal immunotolerance that is different from allergic inflammation.
TSLP strongly activates CD11c+ peripheral DCs to produce TH2-attracting chemokines CCL17 and CCL22.23 Our results also show that the TSLP-primed CD1c+ decidual DCs and CD11c+ moDCs to produce high levels of CCL17, which may attract blood CCR4+CD4+ T cells into the maternal-fetal interface. Human trophoblasts in early pregnancy also secrete CCL17 to regulate the infiltration of CD4+ T cells into the decidua,24 but at significantly lower levels than that of trophoblasts cocultured with decidual DCs. These 2 sources of CCL17 at the maternal-fetal interface may play a similar role in attracting CD4+ T cells into the decidua.
Human trophoblast cells in first trimester pregnancy induce dDCs to release high IL-10 but low TNF-α level, a status protective for pregnancy. IL-10 directly suppresses T cells or indirectly induces tolerogenic DCs and regulatory T cells.25-27 The dDCs in human uterine decidua probably regulate immune responses to both uterine infections and trophoblast cells, through engagement of human inhibitory receptor ILT4 by its natural ligand, HLA-G.28 Nevertheless, how decidual DCs control TH1 or TH2 differentiation is not well understood.
Activated CD4+ cells can differentiate into at least 2 distinct TH phenotypes: TH1 cells producing IFN-γ that orchestrate the T cell–dependent cytotoxicity in certain autoimmune diseases, allograft rejection, and delayed-type hypersensitivity; and TH2 cells secreting IL-4 to facilitate antibody-producing B cells. Certain cross-regulating cytokines are also key in the TH1 and TH2 differentiation from CD4+ T cells. Pregnancy is known to produce a TH2 bias.1,2,29 In mice, TH2 cytokines, such as IL-10 and IL-4, are beneficial, whereas TH1 cytokines, such as IFN-γ and TNF-α, are harmful in fetoplacental growth.
Although activated decidual CD4+ T cells express functional TSLPRs, TSLP does not directly induce TH1 or TH2 differentiation. Rochman et al also reported that human peripheral CD4+ T cells proliferate significantly in response to rhTSLP.30 However, Zhou et al demonstrated that TSLP directly promoted murine CD4+ T cell to differentiate into TH2 cells.31 The TSLPR expression on activated decidual CD4+ T cells is very low; thus, TSLP-TSLPR signaling pathway may be involved in the crosstalk between the decidual DCs and CD4+ T cells, and TH2 cell expansion may contribute to successful pregnancy.
Compared with normal early pregnancy, trophoblast cells from miscarriage express much lower TSLP. In the intestine, low TSLP expression is associated with Crohn disease. However, TSLP overexpression in the skin or bronchial tube leads to atopic dermatitis or asthma.7,31 The local homeostasis of TH1/TH2 is broken when the TSLP expression level is changed, resulting in such TH1-mediated intestinal pathologies or TH2-mediated allergic disease.9,12 We propose that the TH2 bias at the maternal-fetal interface maintained by the TSLP-dDCs is important for maternal tolerance to the fetus during early pregnancy.
The resident dDCs in close contact with trophoblasts are accurately regulated. Trophoblasts expressing paternal antigens are not simply tolerated by the maternal immune system but instead may actively express TSLP and other bioactive molecules to instruct dDCs to induce a favorable TH2-biased immune milieu.9,12,32,33 The embryo-derived trophoblasts may modulate the function of the decidual immunocompetent cells in a DC-dependent manner via TSLP-TSLPR signaling.
Here, we compared the isolated dDCs and in vitro generated moDCs in a series of experiments. Because of the low frequency, direct isolation of sufficient amounts of peripheral CD11c+ DCs from one donor was not possible. Although we have demonstrated TSLP expression in DECs by immunohistochemistry, the primary DECs fail to secrete soluble TSLP. Previous reports suggest that DCs and natural killer (NK) cells may also mediate TSLP-mediated cell signaling and generate a similar Th2-bias status, characterized by expanded IL-10+ NK cells.34,35 This may partially occur because some T cells and certain NK cells share the same progenitors.36,37
Indeed, the Th1/Th2 paradigm in pregnancy is too simplistic, as implantation and early pregnancy are clearly accompanied by inflammatory (ie, Th1) processes that may actually benefit pregnancy.38,39 However, maintaining at least a Th1/Th2 balance is critical to a successful pregnancy. In addition, Th1 bias is commonly recognized at the peri-implantation stage of embryos; and thereafter, the Th2 bias is apparent in the early gestation, which is necessary for fetal and placental development.40
In conclusion, our study shows that trophoblast-derived TSLP induces a classic TH2 bias at human maternal-fetal interface through instructing decidual DCs, which may play an important role in establishing and maintaining maternal-fetal immunotolerance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Basic Research Program of China (2006CB944007), Key Project and Major International Joint Research Project of National Natural Science Foundation of China (30730087, 30910103909), National Natural Science Foundation of China (30670787), National and Shanghai Leading Academic Discipline Project (211XK22), and the Program for Outstanding Medical Academic Leader (all D.-J.L.); and by the Foundation for Young Investigator of Chinese Education Ministry (20070246037; M.-R.D.).
Authorship
Contribution: P.-F.G. and H.-X. W. performed the major part of the experiments and wrote the manuscript; M.-R.D. replenished experiment and revised the manuscript; Y.L. contributed to immunohistochemistry of TH1 and TH2 cytokines and reviewed the manuscript; L.-P. J. contributed to RT-PCR and flow cytometry; and D.-J. L. designed and supervised the study and edited and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Da-Jin Li, Laboratory for Reproductive Immunology, Hospital and Institute of Obstetrics and Gynecology, IBS, Fudan University Shanghai Medical College, Shanghai 200011, China; e-mail: djli@shmu.edu.cn.
References
Author notes
P.-F.G., M.-R.D., and H.-X.W. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal