Abstract
PKCζ has emerged as a pathologic mediator of endothelial cell dysfunction, based on its essential role in tumor necrosis factor α (TNFα)–mediated inflammation. In contrast, extracellular signal–regulated kinase 5 (ERK5) function is required for endothelial cell homeostasis as shown by activation of Krüppel-like factor 2 (KLF2), increased endothelial nitric-oxide synthase (eNOS) expression, and inhibition of apoptosis. We hypothesized that protein kinase C ζ (PKCζ) activation by TNFα would inhibit the ERK5/KLF2/eNOS pathway. TNFα inhibited the steady laminar flow–induced eNOS expression, and this effect was reversed by the dominant-negative form of PKCζ (Ad.DN-PKCζ). In addition, ERK5 function was inhibited by either TNFα or the transfection of the catalytic domain of PKCζ. This inhibition was reversed by PKCζ small interfering RNA. PKCζ was found to bind to ERK5 under basal conditions with coimmunoprecipitation and the mammalian 2-hybrid assay. Furthermore, PKCζ phosphorylates ERK5, and mutation analysis showed that the preferred site is S486. Most importantly, we found that the predominant effect of TNFα stimulation of PKCζ was to decrease eNOS protein stability that was recapitulated by transfecting Ad.ERK5S486A mutant. Finally, aortic en face analysis of ERK5/PKCζ activity showed high PKCζ and ERK5 staining in the athero-prone region. Taken together our results show that PKCζ binds and phosphorylates ERK5, thereby decreasing eNOS protein stability and contributing to early events of atherosclerosis.
Introduction
Endothelial nitric-oxide synthase (eNOS) is a key enzyme involved in the regulation of vascular function, and the altered activity and expression of this enzyme has been shown to contribute to atherosclerosis.1-4 It has been reported that eNOS is regulated at the transcriptional, posttranscriptional, and posttranslational levels.5,6 For example, tumor necrosis factor α (TNFα) has been shown to inhibit eNOS expression by down-regulating both transcriptional and posttranscriptional processes.7-9
Inflammation plays a central role in the pathogenesis of atherosclerosis.10-13 TNFα, in addition to regulating eNOS expression, is a mediator of inflammation, and protein kinase C ζ (PKCζ) is a key enzyme for the TNFα-mediated inflammation. When endothelial cells (ECs) are stimulated by TNFα, PKCζ is activated and promotes monocyte adhesion by increased nuclear factor-κB–dependent intercellular adhesion molecule 1 expression.14,15 In addition, we found that PKCζ activity was required for TNFα-mediated activation of c-Jun N-terminal kinase and caspase-3 in ECs,16 events that cause endothelial dysfunction. Interestingly, increased PKCζ phosphorylation (ie, active form of the enzyme) was found in ECs located in the athero-susceptible region of porcine aorta.17 Together, these observations suggest an important role of PKCζ in the process of atherogenesis by up-regulating inflammatory pathways in ECs.
A distinctive characteristic of PKCζ is the presence at the N-terminus of a novel protein-protein interaction module, termed PB1. The PB1 domain is named after the prototypical domain found in Phox and Bem1p, which mediate polar-heterodimeric interactions.18 This domain is also present in the mitogen extracellular-signal-regulated kinase kinase 5 (MEK5), the upstream activator of the extracellular signal-regulated kinase 5 (ERK5), suggesting that there may be a cross talk between the PKCζ atherogenic and the MEK5-ERK5-KLF2 (Krüppel-like factor 2) atheroprotective signaling pathways. This latter pathway is activated by steady laminar flow (s-flow) and inhibits atherosclerosis.19,20
The atheroprotective effects of s-flow are well known. For example, we have earlier reported that s-flow potently activates ERK521 and that this s-flow–mediated ERK5 activation induces the expression of KLF2, a recently identified transcriptional activator of eNOS and an inhibitor of EC inflammation.22,23 Furthermore, we have shown that peroxisome proliferator activated receptor γ1 is activated by s-flow by way of ERK5 activation and contributes to the overall anti-inflammatory and athero-protective effects of flow.24 In addition to its kinase activity, ERK5 acts as a transcriptional activator. The C-terminus region of ERK5 has 2 transactivation domains, one of them (aa684-806) is constitutively active. Importantly, proatherogenic stimuli inhibit ERK5 activity, in part by stimulating SUMOylation at Lys6 and Lys22, which decreases flow-mediated KLF2 promoter activity and eNOS expression.25
The mechanism by which TNFα decreases eNOS expression in ECs is not fully elucidated. Here, we study the involvement of PKCζ and the MEK5/ERK5 pathway in the TNFα-induced down-regulation of eNOS expression in ECs and in the initiation of atherosclerosis.
Methods
Antibodies, small interfering RNA, adenovirus, and reagents
Antibodies against ERK5 and p-PKCζ were purchased from Cell Signaling; anti-Flag and anti-tubulin from Sigma-Aldrich; anti-PKCζ, anti–hemagglutinin A (HA) and, anti–VP-16 from Santa Cruz Biotechnology Inc (CA); and anti-eNOS and anti– platelet endothelial cell adhesion molecule 1 (PECAM-1) from BD Transduction Laboratories. Peroxidase-conjugated goat anti–mouse and anti–rabbit antibodies were obtained from GE Healthcare. Alexa Fluor 488– or 546–conjugated goat anti–rabbit and anti–rat antibodies were purchased from Molecular Probes. The predesigned and prevalidated human-specific PKCζ small interfering RNA (siRNA; ON-TARGET plus SMART pool PKCζ siRNA; L-003526-00-00) and control siRNA were from Dharmacon RNA Technologies. Adenovirus that drives expression of a dominant-negative form of PKCζ (Ad.PKCζ-DN, ADV-112) was purchased from Cell Biolabs. TNFα was purchased from Roche Applied Science, and active recombinant human PKCζ was obtained from Stressgen Biotechnologies.
Plasmid construction
Mouse ERK5 and the constitutively active form of MEK5α (CA-MEK5α) were cloned as described previously.26 PKCζ wild-type (WT) and catalytic domain (CATζ) constructs were kind gifts from Dr Jae-Won Soh (Columbia University), and eNOS promoter was a gift from Dr David Gardner (University of California, San Francisco). The reporter gene encoding KLF2 promoter (−924 to +14) was a gift from Dr Jerry Lingrel (University of Cincinnati). Gal4-ERK5 and VP16-ERK5 were created by inserting the mouse ERK5 isolated from pcDNA3.1-ERK5 into BamH1 and Not1 sites of the pBIND and pACT vectors, respectively. Single mutations of ERK5 were created with the QuikChange site-directed mutagenesis kit (Stratagene). All constructs were verified by DNA sequencing.
Cell culture and transfection
Human umbilical vein endothelial cells (HUVECs) were obtained from collagenase-digested umbilical veins and collected in M200 medium supplemented with low serum growth supplement (Cascade Biologic), 5% fetal calf serum (GIBCO), 50 U/mL penicillin, and 50 μg/mL streptomycin. Bovine aortic ECs (BAECs) were cultured in M199 medium (GIBCO) supplemented with 10% fetal clone III (Hyclone), minimal essential medium (MEM)–amino acids, 50 U/mL penicillin, and 50 μg/mL streptomycin. HUVECs as well as BAECs were cultured on 2% gelatin precoated dishes. Chinese hamster ovary cells were cultured in F12 medium (GIBCO) supplemented with 10% fetal calf serum (GIBCO), 50 U/mL penicillin, and 50 μg/mL streptomycin. For transient expression experiments, 80% confluent cells were transfected with Opti-MEM and Lipofectamine 2000 (Invitrogen) as previously described.27 Three hours after transfection, Opti-MEM was replaced with complete media, and cells were treated with TNFα 6 hours later.
For siRNA-driven depletion of PKCζ, HUVECs were transiently transfected with 40nM PKCζ or control siRNA with the use of Lipofectamine 2000 reagent. The cells were harvested 36 hours after siRNA transfection. For adenoviral infection, HUVECs were transduced with 50 multiplicity of infection of adenovirus expressing a dominant-negative form of PKCζ (Ad.PKCζ-DN)28 and used 16 hours later.
Mammalian 1- or 2-hybrid analysis and transfection of cells
HUVECs were plated in 12-well plates at 5 × 104 cells/well. For mammalian one-hybrid analysis of transcriptional activity, cells were transfected in Opti-MEM with a Lipofectamine 2000 mixture containing pG5-luc vector, pBIND-ERK5, and pcDNA3 with or without pcDNA3-CA-MEKα. After 3 hours, cells were washed, and fresh medium supplemented with 10% fetal calf serum was added. Cells were treated 8 hours after transfection. For the mammalian 2-hybrid assay, cells were transfected in Opti-MEM with a Lipofectamine mixture containing the pG5-luc vector and various pBIND and pACT plasmids (Promega). Three hours later, Opti-MEM was replaced with complete medium, and cells were incubated overnight, washed twice with phosphate-buffered saline, and lysed in Passive Lysis Buffer (E194A; Promega). Luciferase assays were done with the Luciferase Assay system (E1501; Promega). Luciferase activity was normalized to β-galactosidase activity to correct for differences in transfection efficiency.
eNOS and KLF2 promoter activity
HUVECs were transiently cotransfected with human eNOS promoter (−1197) or KLF2 promoter (−924 to +14) and β-galactosidase with the use of Lipofectamine 2000. Luciferase activity was measured as above.
Steady laminar flow protocol
Confluent cells cultured in 100-mm dishes were exposed to s-flow (shear stress = 12 dyn/cm2) with the use of a cone and plate type of flow apparatus placed in a humidified 5% CO2 incubator at 37°C for 24 hours.
Western blot analysis and immunoprecipitation
Cells were harvested and lysed in RIPA buffer (5mM/L HEPES [N-2-hydroxyethylpiperazine;N′-2-ethanesulfonic acid], 10mM/L EDTA [ethylenediaminetetraacetic acid], 150mM/L NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], pH 7.4) supplemented with the protease inhibitor cocktail.29 Protein concentration was determined by Bradford assay (Bio-Rad), and cell lysates were subjected to SDS–polyacrylamide gel electrophoresis (PAGE). Proteins were then transferred onto nitrocellulose membranes, and the membranes were subsequently blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS)/0.1% Tween20 for 1 hour. After being washed 3 times with PBS/0.1% Tween20, the blots were incubated overnight at 4°C with appropriate primary antibodies. Then, the membranes were incubated with peroxidase-conjugated secondary antibodies for 1 hour. Signals were visualized with the use of the enhanced chemiluminescence Western blotting detection system (Amersham Biosciences). Images were acquired with a Gel Doc System (Gel Doc 2000; Bio-Rad), and a densitometric analysis of membranes was performed using the Bio-Rad software. For the immunoprecipitation analysis, clarified supernatants (400 μg of total protein) were incubated with anti-Flag or anti-ERK5 at 4°C overnight. Lysates were then mixed with 50 μL of protein A/G agarose beads and incubated for 2 hours at 4°C. Immune complexes were collected by centrifugation (3000g for 2 minutes) and washed 4 times with the RIPA buffer, and then bound proteins were released in 2× SDS gel sample buffer. Then, the immunoprecipitates were subjected to SDS-PAGE and Western blot analysis.
In vitro phosphorylation of ERK5 by activated PKCζ
Glutathione-S-transferase (GST)–ERK5–truncated mutant proteins were expressed in Escherichia coli, purified with glutathione-Sepharose 4B as described (Pharmacia Biotech Inc), and used in in vitro kinase assays by activated PKCζ as described previously.26 Briefly, each GST-ERK5 fragment (3 μg) was incubated for 30 minutes at 30°C in a reaction mixture (40 μL) containing 15 μmol/L adenosine triphosphate (ATP), 10mmol/L MgCl2, 10mmol/L MnCl2, 3 μCi (0.111 Bq) of [γ-32P]ATP, and 10 ng of active recombinant human PKCζ. The reaction was terminated by adding 6 μL of 6× electrophoresis sample buffer and boiling for 5 minutes. Samples were analyzed on 10% SDS-PAGE, followed by autoradiography.
Real-time quantitative polymerase chain reaction analysis of eNOS and KLF2
Total RNA was isolated with the use of the TRIazol reagent (Invitrogen) and reverse transcription was conducted with the use of TaqMan reverse transcription reagents (Applied Biosystem) following the manufacturer's instruction. The relative quantities of specific mRNAs were obtained with the use of the comparative Ct method and were normalized to glyceraldehyde-3-phosphate dehydrogenase (Applied Biosystem).
Animal model and en face confocal analysis
All animal experiments were conducted in accordance with experimental protocols that were approved by the Institutional Animal Care and Use Committee at the University of Rochester. ApoE−/− mice on a C57BL/6J background were obtained from The Jackson Laboratory.
Immunofluorescence staining of mouse aortic ECs was performed as described previously.30 Aortas of 12-week-old female ApoE−/− mice were perfused with PBS followed by 2% paraformaldehyde in PBS for 10 minutes. After fixation, the aortas were cut into small fragments and incubated in blocking buffer containing 2% bovine serum albumin and 0.1% Triton X-100 in PBS. Primary antibody incubations were performed overnight at 4°C. After washing the aortic segments 3 times, the secondary antibodies were added and incubated for 1 hour. For negative controls, nonimmune goat or rabbit immunoglobulin G was used in place of primary antibodies. After 3 washes, aortic specimens were opened, placed on a glass slide with the luminal side up, and then mounted for confocal microscopy (Olympus; FLUOVIEW300).
Statistical analysis
Data are shown as mean plus or minus SD for 3 to 4 separate experiments. Differences were analyzed by 1-way analysis of variance or Student t test. P values are expressed as less than .1 and less than .05. The latter was considered statistically significant.
Results
TNFα inhibits eNOS expression by a PKCζ-dependent pathway
To investigate the involvement of PKCζ activation in the TNFα-mediated inhibition of eNOS, we first treated BAECs with TNFα (0-10 ng/mL; 15 minutes), and PKCζ activity was measured by phosphorylation at Thr410 (p-PKCζ) by immunoblotting (Figure 1A-B). There was a significant 3-fold increase in PKCζ activity that was maximal at 5 ng/mL. TNFα treatment did not affect PKCζ expression in these cells (Figure 1A).
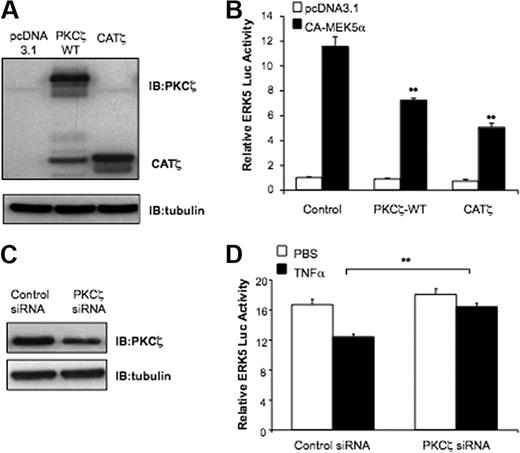
Reducing PKCζ activity by Ad.DN-PKCζ reverses TNFα inhibition of eNOS. (A-B) TNFα increases PKCζ activation/phosphorylation. (A) Confluent BAECs were exposed to TNFα 5 and 10 ng/mL or vehicle alone for 15 minutes. Then, cells were lysed and immunoblotted with p-PKCζ and total PKCζ antibodies, respectively. The amount of proteins loaded in each lane was equal as shown by incubating the same blots with antitubulin antibody. (B) Densitometric analysis of p-PKCζ. Results were normalized by arbitrarily setting the average densitometry of the control to 1.0. Representative blots are shown from 3 separate experiments. *P < .01. (C-D) TNFα-mediated eNOS reduction is PKCζ dependent. (C) HUVECs were transfected with Ad.LacZ (control) or Ad.DN-PKCζ. After 16 hours cells were treated with TNFα or vehicle and then exposed to flow (shear stress = 12 dyn/cm2) for 24 hours. Western blots were performed for eNOS, PKCζ, and tubulin. (D) eNOS expression was analyzed by densitometry and normalized by setting static cells to 1.0. Data are mean ± SD of 3 experiments in triplicate. **P < .05.
Reducing PKCζ activity by Ad.DN-PKCζ reverses TNFα inhibition of eNOS. (A-B) TNFα increases PKCζ activation/phosphorylation. (A) Confluent BAECs were exposed to TNFα 5 and 10 ng/mL or vehicle alone for 15 minutes. Then, cells were lysed and immunoblotted with p-PKCζ and total PKCζ antibodies, respectively. The amount of proteins loaded in each lane was equal as shown by incubating the same blots with antitubulin antibody. (B) Densitometric analysis of p-PKCζ. Results were normalized by arbitrarily setting the average densitometry of the control to 1.0. Representative blots are shown from 3 separate experiments. *P < .01. (C-D) TNFα-mediated eNOS reduction is PKCζ dependent. (C) HUVECs were transfected with Ad.LacZ (control) or Ad.DN-PKCζ. After 16 hours cells were treated with TNFα or vehicle and then exposed to flow (shear stress = 12 dyn/cm2) for 24 hours. Western blots were performed for eNOS, PKCζ, and tubulin. (D) eNOS expression was analyzed by densitometry and normalized by setting static cells to 1.0. Data are mean ± SD of 3 experiments in triplicate. **P < .05.
To study the effect of PKCζ activation on eNOS protein expression we used s-flow (12 dynes/cm2; 24 hours) to stimulate eNOS expression. As shown in Figure 1C and D, s-flow increased eNOS expression by 1.3- plus or minus 0.09-fold (N = 3; P < .05). The addition of TNFα (10 ng/mL; 24 hours) had 2 significant effects on eNOS expression. First, there was a 55% decrease in expression (Figure 1D bars 1 vs 3), and second, the s-flow–mediated increase in eNOS expression (lanes 2 vs 1) was significantly inhibited by TNFα (lanes 4 vs 2). To determine whether this effect could be reversed by inhibiting PKCζ activity, we used the dominant-negative form of PKCζ (ATP binding site K to M mutant; DN-PKCζ). HUVECs were transduced with Ad.DN-PKCζ or adenoviral β-galactosidase (Ad.LacZ) and exposed to s-flow with or without TNFα. HUVECs transduced with Ad.LacZ expressed the same level of eNOS as sham-transfected cells (not shown) and Ad.DN-PKCζ (lanes 5 vs 1). Cells expressing Ad.DN-PKCζ exhibited an s-flow–mediated increase in eNOS expression (1.3-fold ± 0.1-fold; N = 3; P < .05) that did not differ from control Ad.LacZ-expressing cells (Figure 1C-D lanes 6 vs 2). More importantly, Ad.DN-PKCζ transduction significantly reduced the TNFα-mediated inhibition of eNOS expression in HUVECs exposed to s-flow (43% increase; Figure 1C lanes 8 vs 4). These data show that eNOS expression induced by s-flow is negatively regulated by PKCζ activity.
PKCζ activation by TNFα inhibits ERK5 function
The critical role of the MEK5/ERK5/KLF2 pathway in inhibiting endothelial inflammation and stimulating eNOS expression is well described.22,25,31-33 To investigate whether PKCζ activation negatively regulates ERK5, we cotransfected HUVECs with PKCζ-WT, CATζ, or the empty vector (pcDNA3.1) with Gal4-ERK5 and constitutively active MEK5 (CA-MEK5α) constructs as indicated (Figure 2), and then performed a luciferase assay. Both PKCζ-WT and CATζ significantly inhibited CA-MEK5α–mediated ERK5 transactivation (Figure 2B).
TNFα inhibits ERK5 transactivation by PKCζ. (A) HA-PKCζ-WT and CATζ overexpression was verified with the use of cell lysates probed with anti-PKCζ. (B) PKCζ-WT and CATζ inhibit ERK5 transactivation. HUVECs were transfected with pBIND-ERK5 and Gal4-dependent (pG5-Luc) reporter gene with or without pcDNA3-CA-MEK5α with 0.3 μg of control vector or expression plasmids for PKCζ-WT or CATζ. ERK5 transcriptional activity was evaluated by measuring luciferase activity after 24 hours. (C) PKCζ silencing was confirmed by Western blot analysis. (D) Depletion of PKCζ expression reverses TNFα-mediated inhibition of ERK5 transcriptional activity. HUVECs were pretreated for 24 hours with siRNA targeting PKCζ and then cotransfected with pBIND-ERK5 and Gal4-dependent (pG5-Luc) reporter gene with or without pcDNA3-CA-MEK5α. After 8 hours, cells were treated with 10 ng/mL TNFα, and ERK5 luciferase activity was determined 16 hours later. Results are expressed in arbitrary units normalized to the control (set to 1.0 for each experiment). Data are mean ± SD of 3 experiments performed in triplicate. **P < .05.
TNFα inhibits ERK5 transactivation by PKCζ. (A) HA-PKCζ-WT and CATζ overexpression was verified with the use of cell lysates probed with anti-PKCζ. (B) PKCζ-WT and CATζ inhibit ERK5 transactivation. HUVECs were transfected with pBIND-ERK5 and Gal4-dependent (pG5-Luc) reporter gene with or without pcDNA3-CA-MEK5α with 0.3 μg of control vector or expression plasmids for PKCζ-WT or CATζ. ERK5 transcriptional activity was evaluated by measuring luciferase activity after 24 hours. (C) PKCζ silencing was confirmed by Western blot analysis. (D) Depletion of PKCζ expression reverses TNFα-mediated inhibition of ERK5 transcriptional activity. HUVECs were pretreated for 24 hours with siRNA targeting PKCζ and then cotransfected with pBIND-ERK5 and Gal4-dependent (pG5-Luc) reporter gene with or without pcDNA3-CA-MEK5α. After 8 hours, cells were treated with 10 ng/mL TNFα, and ERK5 luciferase activity was determined 16 hours later. Results are expressed in arbitrary units normalized to the control (set to 1.0 for each experiment). Data are mean ± SD of 3 experiments performed in triplicate. **P < .05.
To inhibit PKCζ activity we used siRNA to reduce PKCζ expression (Figure 2C). To assay the effect of reducing PKCζ we measured ERK5-dependent luciferase activity as described in Figure 2B. ERK5 transactivation was inhibited by TNFα in control siRNA transfected cells and reversed in PKCζ siRNA transfected cells (Figure 2D). These data show a critical role for PKCζ in TNFα-induced inhibition of ERK5 transactivation.
We have previously reported that ERK5 SUMOylation at Lys6 and Lys22 inhibited its transcriptional activity.25 These data suggest that the inhibition of ERK5 transactivation by TNFα may be due to ERK5 SUMOylation. To test this possibility, we studied the effect of TNFα on ERK5 transactivation in HUVECs expressing ERK5-K6R/K22R (K6/K22R mutant) which cannot undergo SUMOylation.25 HUVECs were transfected with ERK5-K6/K22R, a Gal4-dependent luciferase reporter gene with or without CA-MEK5α, treated with TNFα, and assayed for luciferase activity. TNFα significantly inhibited ERK5 transactivation in a dose-dependent manner (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), but we observed no significant effects of the K6/K22R mutant on the TNFα-induced inhibition of ERK5 transactivation (supplemental Figure 1B). To determine whether ERK5 tyrosine phosphorylation at the TEY motif (Thr218/Tyr220; the dual-phosphorylation site) is involved in its down-regulation by TNFα, HUVECs were transduced with Ad.CA-MEK5α to activate ERK5. We found no inhibition of ERK5 phosphorylation at these sites by TNFα (supplemental Figure 1C). These results suggest that TNFα-mediated inhibition of ERK5 transactivation is not due to ERK5 SUMOylation or phosphorylation of the TEY motif.
PKCζ associates directly with ERK5
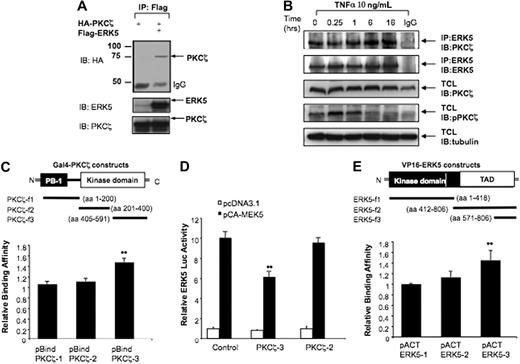
To determine whether PKCζ inhibition involved direct binding to ERK5, we tested their interaction. We cotransfected HeLa cells with HA-tagged PKCζ and Flag-tagged ERK5, immunoprecipitated with Flag antibody, and assayed for HA-PKCζ in the immunoprecipitates (Figure 3A). Under these conditions there was coprecipitation of PKCζ and ERK5. To investigate the interaction between endogenous PKCζ and ERK5, we stimulated HUVECs with TNFα at the indicated times, and the cell lysates were immunoprecipitated with ERK5 antibody (Figure 3B). We found that PKCζ coimmunoprecipitated with ERK5 under basal conditions (T = 0 hours) and that TNFα stimulation did not significantly increase PKCζ-ERK5 interaction (Figure 3B). These results suggest that PKCζ is constitutively associated with ERK5.
PKCζ interacts with ERK5. (A) ERK5 binds to PKCζ in vitro. HeLa cells were cotransfected with HA-PKCζ and pcDNA3 or Flag-ERK5 for 24 hours and subjected to immunoprecipitation with Flag antibody, followed by Western blot analysis with HA antibody. Expression of ERK5 and PKCζ was detected by Western blotting with specific antibodies. (B) TNFα slightly increases PKCζ-ERK5 binding. Subconfluent cocultures of HUVECs were treated for different time points with TNFα 10 ng/mL. The interaction of endogenous PKCζ with endogenous ERK5 was evaluated by immunoprecipitating 400 μg of total cell lysate with ERK5 antibody, and the immunoprecipitates were analyzed by immunoblotting with PKCζ antibody. Essentially identical results were obtained in 2 other experiments. (C,E) The COOH-terminus regions of PKCζ and ERK5 are critical for the ERK5-PKCζ interaction. HUVECs were transfected with plasmids expressing wild-type VP16-ERK5 with Gal4-PKCζ fragments (C) or wild-type Gal4-PKCζ with VP16-ERK5 fragments (E) as indicated, and luciferase activity was evaluated 24 hours after transfection. (D) PKCζ fragment able to bind ERK5 inhibits ERK5 transactivation. HUVECs were transfected with pBIND-ERK5 and Gal4-dependent (pG5-Luc) reporter gene with or without pcDNA3-CA-MEK5α with PKCζ-3 and PKCζ-2 fragments. Luciferase activity was measured after 24 hours of incubation. Data are mean ± SD of 3 experiments performed in triplicate. **P < .05.
PKCζ interacts with ERK5. (A) ERK5 binds to PKCζ in vitro. HeLa cells were cotransfected with HA-PKCζ and pcDNA3 or Flag-ERK5 for 24 hours and subjected to immunoprecipitation with Flag antibody, followed by Western blot analysis with HA antibody. Expression of ERK5 and PKCζ was detected by Western blotting with specific antibodies. (B) TNFα slightly increases PKCζ-ERK5 binding. Subconfluent cocultures of HUVECs were treated for different time points with TNFα 10 ng/mL. The interaction of endogenous PKCζ with endogenous ERK5 was evaluated by immunoprecipitating 400 μg of total cell lysate with ERK5 antibody, and the immunoprecipitates were analyzed by immunoblotting with PKCζ antibody. Essentially identical results were obtained in 2 other experiments. (C,E) The COOH-terminus regions of PKCζ and ERK5 are critical for the ERK5-PKCζ interaction. HUVECs were transfected with plasmids expressing wild-type VP16-ERK5 with Gal4-PKCζ fragments (C) or wild-type Gal4-PKCζ with VP16-ERK5 fragments (E) as indicated, and luciferase activity was evaluated 24 hours after transfection. (D) PKCζ fragment able to bind ERK5 inhibits ERK5 transactivation. HUVECs were transfected with pBIND-ERK5 and Gal4-dependent (pG5-Luc) reporter gene with or without pcDNA3-CA-MEK5α with PKCζ-3 and PKCζ-2 fragments. Luciferase activity was measured after 24 hours of incubation. Data are mean ± SD of 3 experiments performed in triplicate. **P < .05.
To determine the ERK5 binding site for PKCζ, we used a mammalian 2-hybrid assay in HUVECs. Plasmids containing GAL4-DBD and truncated forms of PKCζ were constructed with the use of the pBIND vector. A plasmid containing VP16-ERK5 was constructed with the use of the pACT vector. Transfection of HUVECs with these constructs, and assay for luciferase activity as a measure of interaction, showed that only cells expressing the C-terminal kinase domain (aa405-591) of PKCζ exhibited increased activity (Figure 3C), indicating that this is the ERK5 binding domain. To study further functional interaction, we transfected cells with CA-MEK5α and the truncated PKCζ constructs (Figure 3D). As expected, PKCζ fragment 3 (aa405-591), but not fragment 2 (aa201-400), significantly decreased ERK5 transactivation (Figure 3D).
To determine the PKCζ binding site for ERK5, we cotransfected HUVECs with Gal4-PKCζ and the truncated forms of VP16-ERK5 constructs. The interaction of these fragments was then assayed by luciferase activity. As shown in Figure 3E, we found that the C-terminal domain (aa571-806) of ERK5 was required for PKCζ-ERK5 association. Because this domain has multiple potential protein association motifs, future studies will be required to define the specific regions necessary for interaction.
PKCζ phosphorylates ERK5
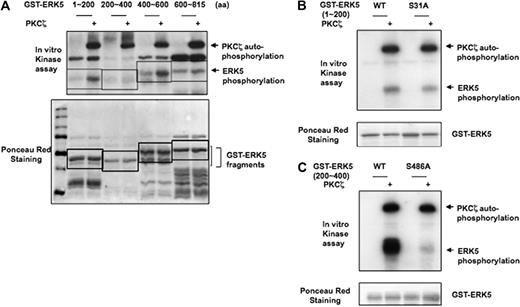
To investigate whether PKCζ phosphorylates ERK5 and, if so, to determine the site(s) of phosphorylation, we generated GST-tagged ERK5 fragments and performed in vitro kinase assay with PKCζ. ERK5 fragments consisting of aa1-200 and aa400-600 were highly phosphorylated by PKCζ (Figure 4A). When we used NetPhosK1.0 software to search for possible phosphorylation sites in the ERK5 sequence, 2 serine residues (S31 and S468) were identified as the most likely phosphorylation targets. GST-ERK5 with either S31A or S486A point mutations were used in the in vitro kinase assay. We found that the S486A point mutation ablated PKCζ-mediated ERK5 phosphorylation (Figure 4C), whereas the S31A mutation did not (Figure 4B). These results indicate that PKCζ phosphorylates S486 of ERK5.
PKCζ directly phosphorylates ERK5 in vitro. (A) To determine direct PKCζ-induced ERK5 phosphorylation, we performed an in vitro kinase assay with 4 different GST-ERK5 fragments as substrate. In vitro kinase assay shows 32P incorporation into 2 GST-ERK5 fragments (amino acids 100 ∼ 200 and 400 ∼ 600) but not into the others (amino acids 200 ∼ 400 and 600 ∼ 816). Ponceau staining shows the position of the proteins after separation by SDS-PAGE and near equal expression. (B-C) Characterization of PKCζ phosphorylation sites. In vitro kinase assay was performed with ERK5-WT fragment (aa1-200 or aa400-600) and ERK5 fragment with S31A (B) or S486A (C) mutations in the presence of recombinant PKCζ. Mutation of the serine 486 to alanine in GST-ERK5 200-600 ablated PKCζ-mediated ERK5 phosphorylation. Data presented are from a representative experiment of at least 3 independent experiments.
PKCζ directly phosphorylates ERK5 in vitro. (A) To determine direct PKCζ-induced ERK5 phosphorylation, we performed an in vitro kinase assay with 4 different GST-ERK5 fragments as substrate. In vitro kinase assay shows 32P incorporation into 2 GST-ERK5 fragments (amino acids 100 ∼ 200 and 400 ∼ 600) but not into the others (amino acids 200 ∼ 400 and 600 ∼ 816). Ponceau staining shows the position of the proteins after separation by SDS-PAGE and near equal expression. (B-C) Characterization of PKCζ phosphorylation sites. In vitro kinase assay was performed with ERK5-WT fragment (aa1-200 or aa400-600) and ERK5 fragment with S31A (B) or S486A (C) mutations in the presence of recombinant PKCζ. Mutation of the serine 486 to alanine in GST-ERK5 200-600 ablated PKCζ-mediated ERK5 phosphorylation. Data presented are from a representative experiment of at least 3 independent experiments.
Role of PKCζ and PKCζ-mediated ERK5 phosphorylation in the regulation of eNOS protein stability
We showed earlier that s-flow–induced up-regulation of eNOS expression was inhibited by TNFα and that this inhibition was abrogated by expressing Ad.DN-PKCζ (Figure 1C-D), indicating that PKCζ is involved in down-regulating eNOS expression. Indeed, when we cotransfected Chinese hamster ovary cells with eNOS cDNA and the constitutively active PKCζ (CATζ), eNOS expression was dose-dependently inhibited (Supplemental Figure 2A-B), thus confirming the inhibitory role of PKCζ in eNOS expression. Because s-flow has been shown to induce eNOS mRNA expression by activating the ERK5/MEF2/KLF2 pathway22 and because activation of PKCζ inhibits ERK5 transactivation (Figure 2B), it is possible that TNFα inhibits KLF2 and eNOS promoter activity by activating PKCζ. To test this possibility, HUVECs were transduced with Ad.DN-PKCζ and transfected with eNOS or pKLF2 promoter, exposed to TNFα or s-flow, and promoter activities were assayed by luciferase activity. In control cells, TNFα reduced eNOS and KLF2 promoter activity and mRNA expression under both static and flow conditions (supplemental Figure 3). Interestingly, expression of Ad.DN-PKCζ did not prevent the down-regulation of eNOS and KLF2 promoter activity and mRNA expression. These data suggest that the recovery of eNOS protein expression by Ad.DN-PKCζ shown in Figure 1C may be due to protein stabilization rather than increased transcription.
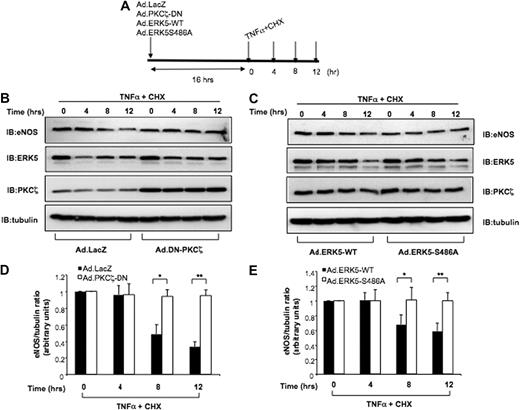
To determine the mechanism by which PKCζ regulates eNOS stability, we first examined TNFα-mediated eNOS degradation by PKCζ activation. HUVECs were transduced with Ad.LacZ or Ad.DN-PKCζ and treated with cycloheximide (CHX) plus TNFα for 0 to 12 hours as indicated (Figure 5A). Then, eNOS expression levels were analyzed by immunoblotting. In control cells expressing Ad.LacZ, TNFα decreased eNOS expression in the presence of the protein synthesis inhibitor CHX, but cells expressing Ad.DN-PKCζ, which inhibits PKCζ activity, significantly prevented the decrease in eNOS protein levels induced by TNFα (Figure 5B,D). These experiments show that PKCζ negatively regulates eNOS protein levels. Next, to determine the role of PKCζ-mediated ERK5 phosphorylation on eNOS stability, we transduced HUVECs with Ad.ERK5-WT or Ad.ERK5-S486 and repeated the same experiments described above. The expression of ERK5-WT and ERK5-S486A mutant was comparable throughout the time course of the experiment (Figure 5C second panel from top). In cells transduced with Ad.ERK5-WT, endogenous eNOS expression decreased after 12 hours of TNFα and CHX treatment. In contrast, in cells expressing the Ad.ERK5-S486A mutant, TNFα and CHX treatment did not significantly decrease eNOS expression by 12 hours (Figure 5C,E). These results suggest that the TNFα-mediated eNOS destabilization depends on PKCζ-mediated ERK5 phosphorylation.
Role of PKCζ in eNOS protein stability. (A) Schematic diagram showing experimental protocol. (B-C) Ad.DN-PKCζ and Ad.ERK5-S486A increase eNOS protein stability. HUVECs were infected with Ad.LacZ (control) or Ad.DN-PKCζ (B) and Ad.ERK5-WT or Ad.ERK5-S486A (C). Sixteen hours later, the cells were incubated with TNFα 10 ng/mL plus the protein synthesis inhibitor cycloheximide (CHX; 10 μg/mL). HUVECs were harvested after 0, 4, 8 and 12 hours of treatment, and Western blots were performed with eNOS, PKCζ, ERK5, and tubulin antibodies. (D-E) Western blots were quantified by densitometry by setting the time zero to 1.0. Data presented are from a representative experiment of at least 3 independent experiments. *P < .1; **P < .05.
Role of PKCζ in eNOS protein stability. (A) Schematic diagram showing experimental protocol. (B-C) Ad.DN-PKCζ and Ad.ERK5-S486A increase eNOS protein stability. HUVECs were infected with Ad.LacZ (control) or Ad.DN-PKCζ (B) and Ad.ERK5-WT or Ad.ERK5-S486A (C). Sixteen hours later, the cells were incubated with TNFα 10 ng/mL plus the protein synthesis inhibitor cycloheximide (CHX; 10 μg/mL). HUVECs were harvested after 0, 4, 8 and 12 hours of treatment, and Western blots were performed with eNOS, PKCζ, ERK5, and tubulin antibodies. (D-E) Western blots were quantified by densitometry by setting the time zero to 1.0. Data presented are from a representative experiment of at least 3 independent experiments. *P < .1; **P < .05.
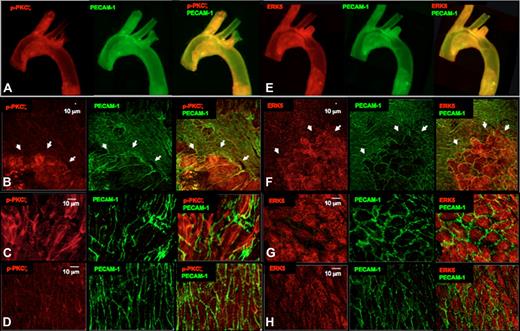
In situ localization of PKCζ and ERK5 in ApoE−/− mouse aorta
Importantly, increased PKCζ activation at disturbed flow (d-flow) areas such as the lesser curvature of the aorta has been observed.17 This athero-prone area is characterized by d-flow in contrast to the relatively protected greater curvature that is characterized by s-flow. Our data provide a novel insight into the mechanism by which eNOS expression is reduced in areas of d-flow as reported.30 Our current study predicts that eNOS degradation in areas of d-flow is the result of phosphorylation of ERK5 by PKCζ. To further elucidate the importance of PKCζ activation in eNOS degradation, we studied an atherosclerotic disease model, the ApoE−/− C57BL/6J mouse. En face preparations of aortas were immunostained for p-PKCζ (Thr410) and PECAM-1 (as an EC marker) and studied with a confocal laser scanning microscope. Low-magnification (4×) images of representative mouse aortas are shown in Figure 6A. There was increased staining for p-PKCζ in atherosclerotic lesions present in the athero-prone region (lesser curvature) compared with athero-protected region (greater curvature). Increased magnification (10×) images show that p-PKCζ was specifically increased in ECs that exhibited abnormal morphology characterized by increased size, loss of polygonal shape, and appearance of gaps between cells (Figure 6B). p-PKCζ appeared to be localized to the perinuclear area and did not colocalize with PECAM-1 (Figure 6B). A direct comparison of the athero-prone region (Figure 6C) with the athero-protected region (Figure 6D) showed that cells with abnormal morphology were the ones that most highly expressed p-PKCζ. Analysis of PECAM-1 showed that it was also more highly expressed in the d-flow region (lesser curvature) than in the s-flow-region (greater curvature). This result is consistent with a recent study indicating that PECAM-1 contributes to atherosclerosis lesion formation in regions of d-flow in ApoE−/− mice.34
PKCζ activation and ERK5 expression in the ApoE−/− mouse. Aortas from 12-week-old ApoE−/− mice were harvested for qualitative analysis of PKCζ activation (A-D red) and ERK5 expression (E-H red). The differential PKCζ activation or ERK5 expression is evident in the images of the whole aortic mount (4× lens; A,E), in the en face analysis of the aortic arch (20× lens; B,F), and specifically in the athero-prone (C,G; 60× lens) and athero-protected (D, H; 60× lens) areas of the aortic arch. EC morphology was changed in the early atherosclerosis regions (B,F; arrowheads) where ECs are stretched and may have lost PECAM-1 staining (green) at some cell junctions. Bar = 10 μm.
PKCζ activation and ERK5 expression in the ApoE−/− mouse. Aortas from 12-week-old ApoE−/− mice were harvested for qualitative analysis of PKCζ activation (A-D red) and ERK5 expression (E-H red). The differential PKCζ activation or ERK5 expression is evident in the images of the whole aortic mount (4× lens; A,E), in the en face analysis of the aortic arch (20× lens; B,F), and specifically in the athero-prone (C,G; 60× lens) and athero-protected (D, H; 60× lens) areas of the aortic arch. EC morphology was changed in the early atherosclerosis regions (B,F; arrowheads) where ECs are stretched and may have lost PECAM-1 staining (green) at some cell junctions. Bar = 10 μm.
Unfortunately, we were unable to develop an antibody that could detect phospho-S486 ERK5, so we studied total ERK5 expression. Analysis of ERK5 expression showed that it was also more highly expressed in athero-prone regions compared with athero-protected regions (Figure 6E). Higher magnification images (Figure 6F) showed that cells that highly expressed ERK5 were the same cells that exhibited abnormal morphology and appeared to overlap with those that highly expressed p-PKCζ (compare Figure 6F with 6B). Interestingly ERK5 appeared to be cytoplasmic as well as nuclear in both normal- and abnormal-appearing ECs (Figure 6G). Direct comparison of the athero-prone region (Figure 6G) to the athero-protected region (Figure 6H) showed no obvious difference in ERK5 subcellular localization. There was clearly a significant increase in ERK5 expression in the d-flow region that corresponded to the increase in p-PKCζ (compare Figure 6G with 6C). These data suggest a potential proatherogenic role for endothelial PKCζ activation and PKCζ-mediated phosphorylation of ERK5.
Discussion
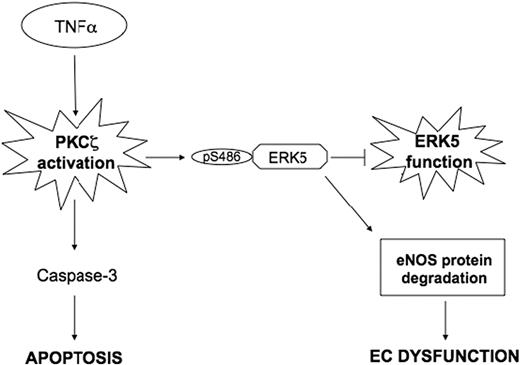
The main finding of the present study is that activation of PKCζ phosphorylates ERK5 and by inhibiting ERK5 function decreases eNOS protein expression in ECs. Specifically, we demonstrated that PKCζ binds and phosphorylates ERK5 at S486, and these events are required to increase eNOS protein degradation (Figure 7). Furthermore, we observed in vivo that PKCζ activity was up-regulated in athero-prone regions of the mouse aorta exposed to d-flow. These results define a new mechanism for endothelial dysfunction and atherosclerosis progression.
A scheme describing the PKCζ-mediated cross talk between the TNFα (proinflammatory) and ERK5 (anti-inflammatory) pathways.
A scheme describing the PKCζ-mediated cross talk between the TNFα (proinflammatory) and ERK5 (anti-inflammatory) pathways.
PKCζ activity was shown to be up-regulated in the d-flow region of the pig aorta by Magid and Davies,17 but the mechanism by which PKCζ contributed to atherosclerosis susceptibility was not described. Previously, we found that PKCζ mediated TNFα-dependent EC apoptosis and was required for activation of caspase-3.16 Recently, eNOS expression was shown to be positively regulated in ECs by a MEK/ERK5/KLF2 pathway.22 Because we demonstrated that ERK5 was activated by s-flow and protected ECs from apoptosis,35 we hypothesized that PKCζ might negatively regulate ERK5 and thereby decrease eNOS expression. Furthermore, because both PKCζ and MEK5 contain a PB1 domain, a well-characterized protein-protein interaction domain, we expected that PKCζ inhibition might be by a direct effect on MEK5. However, we found that PKCζ bound directly to ERK5. Furthermore, the ERK5 binding site was within the catalytic domain of PKCζ, not the PB1 domain. Interestingly, we found that ERK5 is not only a binding partner of PKCζ but is also a substrate of this kinase. Our mutational analysis showed that PKCζ phosphorylates ERK5 at a previously uncharacterized phosphorylation site (S486). Phosphorylation of S486 was necessary for the decrease in eNOS protein stability, although the precise mechanism will require future studies.
We used TNFα as an agonist to stimulate ECs because TNFα has been shown to play an important role during the inflammatory process of atherosclerosis. In particular, TNFα deficiency retards fatty-streak lesion formation by down-regulating the expression of proatherogenic inflammatory factors.36 TNFα has been detected in atherosclerotic lesions throughout all stages of human atherosclerosis33,37 and was found to be associated with atherosclerosis in mouse models.38,39 Mice deficient in both ApoE−/− and TNFα displayed less advanced atherosclerosis than ApoE−/− mice.38,40 In addition, many features of EC dysfunction are mimicked by the inflammatory cytokine TNFα. For example, TNFα stimulation activates PKCζ,15 induces adhesion molecule expression,41 and decreases eNOS expression.7-9 The mechanism for TNFα-mediated inhibition of eNOS expression has been thought to be primarily transcriptional.7,8 However, a role for a decrease in mRNA stability has also been elucidated.9 Surprisingly, our data predict that a posttranslational modification of eNOS protein by PKCζ targets eNOS for degradation. Several mechanisms have been proposed for eNOS degradation with calcium-dependent calpain-mediated degradation being most prominent.42,43 Further studies will be required to define the molecular nature of the PKCζ-ERK5 pathway described here.
To understand the importance of the proposed PKCζ-ERK5 mechanism during early atherosclerotic events, we performed en face staining of aortas from ApoE−/− mice. Specifically, we showed that endothelial PKCζ phosphorylation and activation were increased in the athero-susceptible region of the aortic arch (d-flow region). In the in vivo system of the chow fed ApoE−/− mouse, the endothelium in the lesser curvature of the aorta had distinctively large ECs filled with vacuoles that were positive for p-PKCζ immunostaining. These EC changes, which were probably a consequence of intimal leukocyte accumulation, were not observed in regions that experienced s-flow. Note that the expression of ERK5, which has athero-protective effects, was also up-regulated in the same ECs that showed the highest level of p-PKCζ, suggesting a potential compensatory effect. This finding is consistent with the observations of Passerini et al44 who observed by microarray analysis of athero-prone regions that both atherosclerosis-susceptible (proinflammatory, prothrombotic) and atherosclerosis-protective (antioxidant and antithrombotic) gene expression were up-regulated.
In conclusion, we believe that PKCζ is a proatherogenic effector in ECs and that it could be an innovative therapeutic target to improve endothelial dysfunction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Maria Antonietta Belisario for her support and valuable advice. We thank the Aab Cardiovascular Research Institute members for useful suggestions, in particular Dietrich Machleder, Eugene Chang, and Weiye Wang for great help and Tamlyn Thomas, Chelsea Wong, and Thomas Spangenberg for technical assistance.
This work was supported by National Institutes of Health (grants HL-064839 and HL-077789, B.C.B.; HL-088637, HL-064839, and HL-077789, J.A.; and HL-064839 and HL-077789, K.F.); the internal grant of the University of Salerno (P.N.); and the American Heart Association (postdoctoral fellowship 0625957T, Scientist Development Grant 0930360N; C.-H.W.).
National Institutes of Health
Authorship
Contribution: P.N. contributed to the design of the experiments, performed the experiments, and generated the manuscript and figures; J.A. and K.F. contributed to discussions and design of experiments and critically edited the paper; C.-H.W. helped the design of the experiments and performed experiments; K.S., H.L., J.-D.L., and K.-S.H. contributed to discussions; C.M. and J.-H.L. performed experiments; M.R.O. performed mouse colony management and genotyping; and B.C.B. supervised the project, contributed to the design of the experiments, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bradford C. Berk, Aab Cardiovascular Research Institute, 601 Elmwood Ave, Box CVRI, University of Rochester School of Medicine and Dentistry, Rochester, NY 14642; e-mail: bradford_berk@urmc.rochester.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal