Abstract
β2-Glycoprotein I (β2GPI) is an evolutionary conserved, abundant circulating protein. Although its function remains uncertain, accumulated evidence points toward interactions with endothelial cells and components of the coagulation system, suggesting a regulatory role in vascular biology. Our group has shown that thioredoxin 1 (TRX-1) generates free thiols in β2GPI, a process that may have a regulatory role in platelet adhesion. This report extends these studies and shows for the first time evidence of β2GPI with free thiols in vivo in both multiple human and murine serum samples. To explore how the vascular surface may modulate the redox status of β2GPI, unstimulated human endothelial cells and EAhy926 cells are shown to be capable of amplifying the effect of free thiol generation within β2GPI. Multiple oxidoreductase enzymes, such as endoplasmic reticulum protein 46 (ERp 46) and TRX-1 reductase, in addition to protein disulfide isomerase are secreted on the surface of endothelial cells. Furthermore, one or more of these generated free thiols within β2GPI are also shown to be nitrosylated. Finally, the functional significance of these findings is explored, by showing that free thiol–containing β2GPI has a powerful effect in protecting endothelial cells and EAhy926 cells from oxidative stress–induced cell death.
Introduction
Thioredoxin 1 (TRX-1) is a ubiquitous, 12-kDa oxidoreductase enzyme whose multiple intracellular functions include reducing protein disulfides, scavenging oxygen free radicals, and redox regulation of signaling pathways involved in cell growth, apoptosis, and inflammation.1 TRX-1 requires nitrosylation of the unpaired cysteine (Cys69) thiol to mediate key intracellular functions.2 S-nitrosylation of thiols has been implicated as being important in several multisystemic physiologic and pathologic processes pertaining to hemostasis and vascular disease.3 In addition to being a key intracellular molecule, TRX-1 is an enzyme also found in circulating plasma. It is released from cells in response to oxidative stress4,5 and is found at higher levels in the circulation in a variety of acute and chronic inflammatory or metabolic conditions associated with an increase in systemic oxidative stress load.6-8 Studies have shown that exogenous administration of TRX-1 exerts a protective role in animal models of high oxidative stress such as smoking and rheumatoid arthritis,9,10 both of which represent risk factors for cardiovascular morbidity in humans.11 Oxidative stress is known to be associated with vascular pathology in terms of promoting atherosclerotic plaque development, rupture and atherothrombosis.12 However, the extracellular mechanism through which elevated TRX-1 may mediate a protective effect against oxidative stress–induced vascular injury has not been fully delineated.
β2-Glycoprotein I (β2GPI) is an evolutionary conserved 50-kDa plasma protein. The function of β2GPI remains uncertain, although it is thought to play a role in apoptotic cell clearance and coagulation through multiple interactions with serine proteases, anionic phospholipid, and cell-surface receptors.13-20 It is found in relatively high concentrations in circulating plasma (4μM) and is also found within atherosclerotic plaque lesions,21 although the function of β2GPI relating to atherosclerosis has not been elucidated. The antiphospholipid syndrome is an autoimmune condition characterized by pathogenic circulating anti-β2GPI antibodies. This syndrome, in addition to being associated with vascular thrombosis and recurrent miscarriages, is also associated with accelerated atherosclerosis and enhanced oxidative stress.22-24
Studying the crystal structure of β2GPI in detail shows that the molecular configuration of the Cys288 to Cys326 disulfide bond in the C-terminal domain (domain V) has a unique configuration, with marked surface exposure of Cys326.25,26 Such a configuration may be associated with participation in thio-disulfide exchange reactions with oxidoreductases. This report describes for the first time with the use of human and murine ex vivo experiments that circulating β2GPI contains free thiols. The relevance to endothelial vascular biology is shown through the observation of constitutive secretion of multiple oxidoreductases to the surface of endothelial cells which modulates the free thiol content of TRX-1–treated β2GPI. Nitrosylation of one or more of the free thiols generated within TRX-1–treated β2GPI is also shown. Finally, functional studies show a marked propensity for free thiol–containing β2GPI to protect endothelial cells and EAhy926 cells from oxidative stress–induced cell injury.
Methods
Chemicals and reagents
This information is in the supplemental data available on the Blood Web site (see the Supplemental Materials link at the top of the online article).
Proteins
This information is in the supplemental data.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting
Information about sodium dodecyl sulfate–polyacrylamide gel electrophoresis and Western blotting is detailed in the supplemental data.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were isolated and cultured as described previously.27 The harvesting and use of HUVECs was approved by the Human Research Ethics Committee of St George Hospital, University of New South Wales (UNSW). Informed consent was taken before each cord collection in accordance with the Declaration of Helsinki. The human cell line EAhy926 is derived by fusion of HUVECs and the A549 carcinoma cell line (kind gift from Dr C. J. S. Edgell, University of North Carolina, Chapel Hill).28 Detailed methodology of cell culture is described in the supplemental information.
Generation and 3-(N-maleimidylpropionyl) biocyatin labeling of free thiols in β2GPI
All chemical reactions were performed under argon in 20mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer containing 1.5mM CaCl2, 4mM KCl, 0.5mM Na2HPO4, pH 7.4 (HBS). This was performed as described29 and outlined in the supplemental data.
Effect of human endothelial cells and EAhy926 cells on TRX-1–induced free thiol content within β2GPI
EAhy926 cells were seeded as described in “Cell culture” and washed twice with prewarmed Dulbecco minimal essential medium (DMEM) supplemented with bovine serum albumin (BSA) 0.05%, as were empty wells. Recombinant (rβ2GPI) or native (nβ2GPI; 1μM) preincubated with TRX-1 (3.5μM) activated with dithiothreitol (DTT; 70μM) or TRX-1 reductase (TRX-R; 10nM) plus nicotinamide adenine dinucleotide phosphate (NADPH; 200μM) was then added to EAhy926 cells or to the empty wells (as a control) and incubated at 37°C in a humidified atmosphere of air/CO2 for 5 to 15 minutes. Either 30 μL or 400 μL/well of the β2GPI/TRX-1 mixture was added to 96- or 6-well plates, respectively. The mixture was then transferred to a 500-μL or 1.5-mL Eppendorf tube, incubated with MPB, and quenched with reduced glutathione as described in the supplemental data. The samples were then transferred to polyvinylidene difluoride (PVDF) membranes and probed with streptavidin–horseradish peroxidase or primary antibody of choice. The method used for HUVEC experiments was identical except for the initial wash, which was twice with prewarmed M199 supplemented with 0.05% BSA. Each membrane was stripped and then reprobed with an anti–TRX-1 antibody to quantitate TRX-1 protein, thus confirming equal loading of the protein mixture in each lane. Direct quantification of β2GPI protein loading with the use of anti-β2GPI could not be used for this purpose because pretreatment of β2GPI with activated TRX-1 altered its immunoreactivity to both polyclonal and monoclonal anti-β2GPI antibodies.29
For pull-down experiments, rβ2GPI/TRX-1/DTT mixture after incubation with EAhy926 cells was removed, labeled with MPB as described earlier, and subjected to nickel chromatography (described in detail in the supplemental data). The eluted material was transferred to PVDF membranes and probed with streptavidin–horseradish peroxidase. A bicinchoninic acid protein assay was performed to ensure equal loading. Purity was also confirmed by Coomassie staining of the sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel.
Quantitation of MPB-labeled proteins
Quantitation of MPB-labeled proteins was performed by densitometry image analysis as described in detail in the supplemental data.
Transcript profiling of constitutive oxidoreductase production in HUVECs
A microarray screening approach was used to identify constitutively expressed oxidoreductase transcripts in HUVECs. This was performed as described previously30 and as described in brief in the supplemental data.
Biotin switch to detect nitrosylated cysteines in β2GPI
In addition to using a specific anti–S-nitrosocysteine antibody as stated earlier, nitrosylation of free thiols within rβ2GPI was also detected with the use of a modified version of the established biotin switch method. This is based on the technique described by others previously and is outlined in the supplemental data.31
Hydrogen peroxide treatment of human endothelial and EAhy926 cells and assessment of cell viability
EAhy926 cells were grown to confluence in a 96-well plate as indicated earlier, washed twice in DMEM/BSA 0.05% and then HBS with or without nβ2GPI (2μM) with or without TRX-1 (1.75μM) with or without DTT (35μM; 100 μL/well) was added and incubated at 37°C in a humidified atmosphere of air/CO2 for 20 minutes. The incubation mixtures were then transferred to a 1.5-mL Eppendorf tube containing H2O2 (13mM final) diluted in HBS/0.1% BSA. This solution was reapplied (100 μL/well) to the endothelial cells and incubated at 37°C for a further 20 minutes. Catalase (100 μL; 900 000 U/mL), which degrades H2O2, was then added to each well, and the plates were incubated for a further 2 minutes at 37°C. The cells were then washed twice with DMEM/BSA 0.05% and incubated in DMEM/fetal calf serum (10%)/HBS overnight at 37°C. Analysis of cell viability was then determined with the use of the Promega Cell Titer 96 AQueous One Solution Reagent assay according to the manufacturer's instructions. Absorbance was recorded at 490 nm with a microplate reader PowerWave Microplate Spectrophotometer (BIO-TEK Instruments Inc) read at 4 hours. The absorbance at 490 nm was directly proportional to the number of live cells with the linear range of this assay for EAhy926 cells estimated to be between 5 × 102 and 3 × 104 and for HUVECs between 1 × 103 and 8 × 104 cells per well.27 Cell viability percentage was calculated as follows: (absorbance treated − absorbance media only)/(absorbance untreated control − absorbance media only) × 100. Experiments with HUVECs were based on the methodology described for EAhy926 cells and in the supplemental data.
Assay for in vivo detection of β2GPI with free thiols
Blood collection.
Fully informed consent was obtained before venipuncture, in accordance with ethical standards and procedures of St George Hospital Ethics Committee, UNSW and in accordance with the Declaration of Helsinki. Samples from 18 healthy volunteers were taken (9 females, 9 males; median age, 37.2 years; range, 14-66 years). β2GPI-null mice were generated as described previously.32 Blood was collected by direct cardiac puncture from C57BL/6/β2GPI+/+ and C57BL/6/β2GPI−/− mice (4 mice/group; each group age- and sex-matched: 9 months, 2 males and 2 females for each group). Serum was pooled for each group and stored at −80°C. All animal procedures were conducted with the approval of the UNSW Animal Care and Ethics Committee.
Enzyme-linked immunoabsorbent assay to detect free thiols within β2GPI in vivo.
Human serum samples (50 μL; n = 18) were incubated with or without 4mM MPB for 30 minutes at room temperature (RT) in the dark, under argon with agitation. Serum proteins were then diluted (100-fold for human and murine samples) in 20mM HEPES buffer (pH 7.4) and further incubated at RT for 5 minutes. Proteins were then acetone precipitated to remove the MPB. Protein pellets were resuspended (to the original volume before acetone precipitation) in phosphate-buffered saline (PBS)–Tween (0.05%) and added to a streptavidin 96-well plate (Nalge Nunc International) 100 μL/well in duplicate and incubated at RT for 90 minutes. Before adding MPB-labeled serum samples, streptavidin plates were washed 3 times with PBS-Tween (0.05%) and blocked with 2% BSA/PBS-Tween (0.1%) at RT for 90 minutes. After washing 3 times with PBS-Tween (0.1%), the monoclonal murine anti-β2GPI antibody directed to domain I of β2GPI (clone 4B2E7) was added (100nM) and incubated at RT for 1 hour. For assays that used murine serum, the rabbit polyclonal anti-β2GPI antibody (100nM) was used. After washing 3 times with PBS/Tween (0.1%), goat anti–mouse or anti–rabbit immunoglobulin conjugated to alkaline phosphatase was added (1:1500 dilution; at RT for 1 hour), and samples were read after adding appropriate chromogenic substrate. Absorbance readings were taken at 405 nm. β2GPI-deficient murine (β2GPI−/−) serum and non–MPB-labeled serum and either murine immunoglobulin G2 isotype antibody or rabbit polyclonal immunoglobulin G primary antibodies were used as controls. Determination of coefficient of variation (CV) for this enzyme-linked immunoabsorbent assay (ELISA) was calculated as described in the supplemental data.
Statistical analysis
Comparison in optical density (OD) reading of MPB-labeled versus non–MPB-labeled human serum was performed with the Mann-Whitney test. If more than 2 groups were being compared, then statistical analysis was performed with the use of 1-way analysis of variance followed by Tukey test for comparison of multiple groups or Bonferroni test for comparison of pairs. For microdensitometric data analysis a 2-tailed, unpaired Student t test was used. Significance was denoted by P values less than or equal to .05.
Results
β2GPI within serum ex vivo may be labeled with a free thiol–binding reagent
We have shown that β2GPI treated with TRX-1 generates free thiols within β2GPI and have linked this functionally to platelet adhesion. However, β2GPI could not be labeled with the free thiol–binding reagent MPB, indicating no free thiols in the purified protein.29 To underline the relevance of these and future studies pertaining to the redox status of β2GPI, evidence for the natural occurrence of free thiols within β2GPI in vivo needed to be shown. To further this aim, a novel ELISA method was developed whereby serum is incubated with the biotinylated thiol binding reagent MPB. After removal of free MPB with the use of acetone precipitation, biotinylated serum proteins are incubated with a streptavidin-coated plate, and the sample was then probed for β2GPI with the use of a specific monoclonal anti-β2GPI antibody. The intraplate CV for this ELISA was 5.08% plus or minus 3.09% (mean ± SD; n = 6) and interplate CV was 6.25% (n = 6).
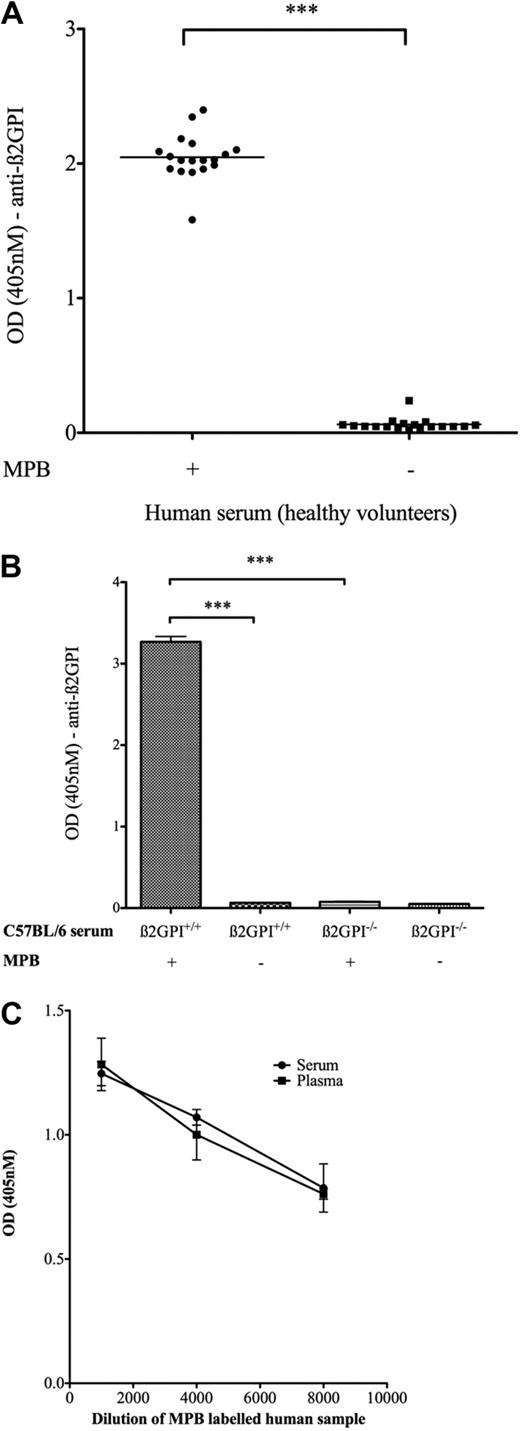
Serum samples from 18 healthy donors were labeled with MPB and analyzed for β2GPI containing free thiols. With the use of this technique, it was shown for the first time that β2GPI within human blood ex vivo can be labeled with MPB (Figure 1A). Controls included non–MPB-labeled human serum probed with anti-β2GPI and both MPB- and non–MPB-labeled serum incubated with an isotype murine control antibody. All such controls showed a negligible signal (OD [405 nm] < 0.1). However, to be certain of the β2GPI specificity of this assay, a murine β2GPI−/− mouse was used as the ideal negative control. With the use of an affinity-purified polyclonal rabbit anti-β2GPI antibody that reacts with murine β2GPI and performing the same ELISA with the murine β2GPI+/+ and β2GPI−/− serum shows a strong signal with MPB-labeled murine β2GPI+/+ serum (mean OD ± SD, 3.27 ± 0.09) but a significantly reduced signal versus MPB-labeled β2GPI−/− serum (0.08 ± 0.007; P ≤ .001; n = 2), shown in Figure 1B.
Evidence for naturally occurring free thiols in β2GPI in both serum and plasma. (A) Human serum samples (n = 18) were incubated with and without MPB, subjected to acetone precipitation (to remove free MPB), incubated on a streptavidin plate, and probed with the murine monoclonal anti-β2GPI antibody 4B2E7. MPB- and non–MPB-labeled samples probed with a murine immunoglobulin G2 isotype control consistently showed low background OD (405 nm) ≤ 0.1. (B) C57BL/6 β2GPI+/+ and β2GPI−/− murine sera (pooled samples; n = 4 mice in each group) were incubated with and without MPB and probed with a polyclonal rabbit anti-β2GPI antibody. (C) Serum and plasma drawn from the same venipuncture (healthy volunteer, age 37 years) were labeled with MPB, and the amount of reduced β2GPI was quantified with the use of the assay described above. Both serum and plasma samples gave the same readings as to amount of reduced β2GPI present. ***P < .001.
Evidence for naturally occurring free thiols in β2GPI in both serum and plasma. (A) Human serum samples (n = 18) were incubated with and without MPB, subjected to acetone precipitation (to remove free MPB), incubated on a streptavidin plate, and probed with the murine monoclonal anti-β2GPI antibody 4B2E7. MPB- and non–MPB-labeled samples probed with a murine immunoglobulin G2 isotype control consistently showed low background OD (405 nm) ≤ 0.1. (B) C57BL/6 β2GPI+/+ and β2GPI−/− murine sera (pooled samples; n = 4 mice in each group) were incubated with and without MPB and probed with a polyclonal rabbit anti-β2GPI antibody. (C) Serum and plasma drawn from the same venipuncture (healthy volunteer, age 37 years) were labeled with MPB, and the amount of reduced β2GPI was quantified with the use of the assay described above. Both serum and plasma samples gave the same readings as to amount of reduced β2GPI present. ***P < .001.
ELISA for circulating reduced β2GPI gives comparable results for both serum and plasma
To ensure that the redox state of β2GPI detected by this novel assay was not specific to serum, we also assessed plasma to determine whether free thiol labeling of β2GPI could be achieved and to assess whether levels differed between the 2 preparations. To test this, serum and plasma were prepared from the same patient (healthy male, 37 years of age) from the same venipuncture, and a reduced β2GPI assay was performed on both samples in serial dilutions in parallel. Figure 1C shows that almost identical levels of reduced β2GPI are obtained for both serum and plasma.
Free thiol generation within β2GPI is amplified by human endothelial and EAhy926 cells
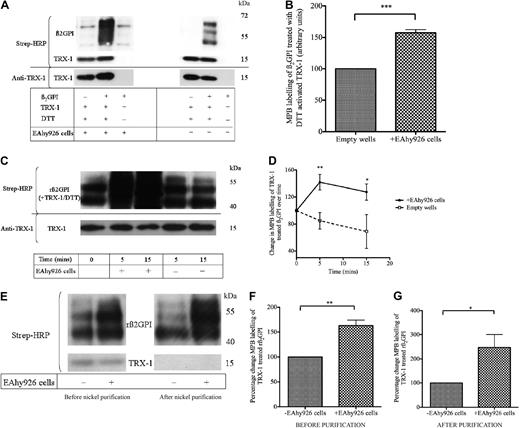
Endothelial cells are known to interact with β2GPI as well as secrete oxidoreductase enzymes such as protein disulfide isomerase (PDI),16,33-35 which we have shown can also generate free thiols within β2GPI.29 Given these observations, the potential of endothelial and EAhy926 cells to modify the redox status of β2GPI was assessed. As shown in Figure 2A and B, nβ2GPI (1μM) preincubated with DTT-activated TRX-1 and then incubated with EAhy926 cells resulted in a significant enhancement of MPB labeling of TRX-1–treated nβ2GPI (57.4% ± 9.7%; n = 4; P ≤ .001) compared with nβ2GPI/TRX-1/DTT incubated with empty wells, washed, and treated in parallel to cell-coated wells. A time course experiment was then performed with rβ2GPI. This showed that the EAhy926 cell mediated amplification of the MPB labeling of TRX-1–treated rβ2GPI also occurs with the recombinant protein and that this amplification takes place within 5 minutes (increase over empty wells was 57.14% ± 23.1%; n = 4; P ≤ .007) and was maintained at 15 minutes (Figure 2C-D). However, TRX-1–treated rβ2GPI incubated in wells without cells had a marked reduction in MPB labeling over time (Figure 2D), indicating that without the EAhy926 cells, free thiols generated within rβ2GPI by activated TRX-1 become rapidly reoxidized over time.
Free thiol formation within β2GPI is enhanced within minutes on incubation with EAhy926 cells. (A-B) TRX-1/DTT–treated nβ2GPI incubated with EAhy926 cells results in an increase in MBP labeling compared with TRX-1/DTT–treated nβ2GPI incubated with empty wells (P ≤ .001; n = 4). (C-D) rβ2GPI (1μM) was pretreated with DTT-activated human TRX-1 (1.75μM) and then incubated in wells coated with and without EAhy926 cells for 0, 5, and 15 minutes. The rβ2GPI/TRX-1 mixture was then labeled with MPB after each respective incubation time. EAhy926 cells enhanced MPB labeling of TRX-1/DTT–treated rβ2GPI within 5 minutes (**P ≤ .007; n = 4) and was maintained at 15 minutes (*P ≤ .04; n = 4). (E) The rβ2GPI/TRX-1/DTT MPB-labeled mixture was then subjected to nickel chromatography, and the degree of relative MPB labeling of equal amounts of purified rβ2GPI from cell-coated and empty wells (750 ng of protein/lane) was determined with streptavidin–horseradish peroxidase (HRP). The loss of MPB-labeled TRX-1 after nickel purification confirms the efficiency of the rβ2GPI purification process. (F-G) The increase in MPB labeling of TRX-1/DTT–treated his-tagged rβ2GPI after cell incubation before nickel purification (**P ≤ .004; n = 3) is also observed after nickel purification (*P ≤ .04; n = 3). Membranes were stripped and probed with anti–TRX-1 to ensure equal protein loading between wells. rβ2GPI versus native β2GPI is approximately 7 kDa smaller because of nonmammalian glycosylation by insect cells.
Free thiol formation within β2GPI is enhanced within minutes on incubation with EAhy926 cells. (A-B) TRX-1/DTT–treated nβ2GPI incubated with EAhy926 cells results in an increase in MBP labeling compared with TRX-1/DTT–treated nβ2GPI incubated with empty wells (P ≤ .001; n = 4). (C-D) rβ2GPI (1μM) was pretreated with DTT-activated human TRX-1 (1.75μM) and then incubated in wells coated with and without EAhy926 cells for 0, 5, and 15 minutes. The rβ2GPI/TRX-1 mixture was then labeled with MPB after each respective incubation time. EAhy926 cells enhanced MPB labeling of TRX-1/DTT–treated rβ2GPI within 5 minutes (**P ≤ .007; n = 4) and was maintained at 15 minutes (*P ≤ .04; n = 4). (E) The rβ2GPI/TRX-1/DTT MPB-labeled mixture was then subjected to nickel chromatography, and the degree of relative MPB labeling of equal amounts of purified rβ2GPI from cell-coated and empty wells (750 ng of protein/lane) was determined with streptavidin–horseradish peroxidase (HRP). The loss of MPB-labeled TRX-1 after nickel purification confirms the efficiency of the rβ2GPI purification process. (F-G) The increase in MPB labeling of TRX-1/DTT–treated his-tagged rβ2GPI after cell incubation before nickel purification (**P ≤ .004; n = 3) is also observed after nickel purification (*P ≤ .04; n = 3). Membranes were stripped and probed with anti–TRX-1 to ensure equal protein loading between wells. rβ2GPI versus native β2GPI is approximately 7 kDa smaller because of nonmammalian glycosylation by insect cells.
Experiments were then performed with the aim of ensuring that the dominant MPB-labeled heterogeneous band migrating at approximately 50 to 70 kDa represented β2GPI and not an irrelevant protein with free thiols of the same size released by the cells. As shown in Figure 2E, rβ2GPI preincubated with DTT-activated TRX-1 and then incubated with EAhy926 cells resulted in significantly enhanced MPB labeling of rβ2GPI (63.1% ± 18.8%; n = 3; P ≤ .004) compared with rβ2GPI/TRX-1 incubated with empty wells washed and treated in parallel to cell-coated wells. The molecular weight of MPB-labeled rβ2GPI (pretreated with activated TRX-1) was heterogeneous (∼43-60 kDa) and as expected approximately 7 kDa smaller than nβ2GPI because of nonmammalian glycosylation by insect cells. With the use of the C-terminal hexahistidine tag of rβ2GPI, the supernatant was then subjected to nickel chromatography to purify rβ2GPI from the protein mixture. A Western blot of the supernatant after nickel purification confirms that this heterogeneous biotin-labeled band indeed represents labeled rβ2GPI. Enhanced labeling of rβ2GPI after incubation with EAhy926 cells is also observed after nickel purification (147.1% ± 91.9%; n = 3; P ≤ .04).
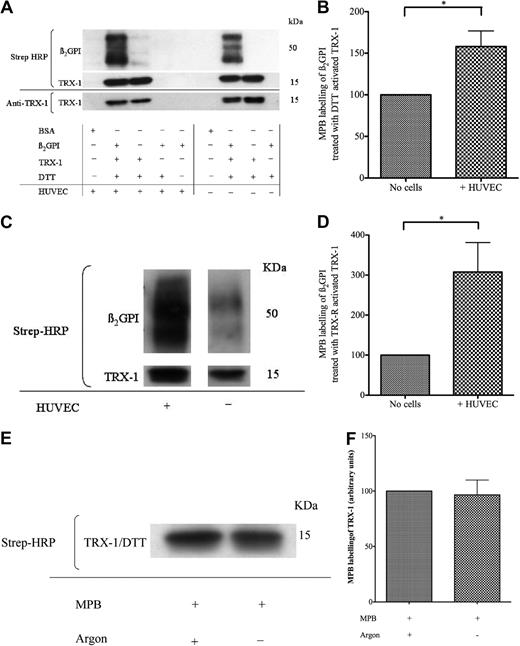
EAhy926 cells are immortalized cells derived from a hybrid of HUVECs and carcinoma cells. The effect of EAhy926 cell–mediated free thiol amplification within TRX-1–treated nβ2GPI was also observed with primary HUVECs (Figure 3A-B). Furthermore, this effect was observed when TRX-1 was activated by the more physiologic method that used TRX-R/NADPH instead of DTT (35μM), as shown in Figure 3C and D.
Amplification of free thiol content within TRX-1–treated nβ2GPI by HUVECs. (A-B) nβ2GPI (1μM) was pretreated with DTT (35μM) activated TRX-1 (1.75μM) for 1 hour and then incubated with HUVECs or empty wells. (C-D) rβ2GPI (1μM) was pretreated with TRX-R (10nM)/NADPH (200μM) activated TRX-1 (1.75μM) for 1 hour and then incubated with HUVECs or empty wells. The supernatant from each well was then labeled with MPB, transferred to a PVDF membrane, and probed with streptavidin–horseradish peroxidase (HRP). This confirmed that HUVECs are also capable of amplifying the free thiol content of (A-B) nβ2GPI pretreated with DTT-activated TRX-1 (mean enhancement ± SD, 58.1% ± 32.5%; *P ≤ .04; n = 3) and (C-D) rβ2GPI pretreated with TRX-R/NADPH–activated TRX-1 (207.6% ± 146.4%; *P ≤ .03; n = 4). Efficiency of MPB labeling of DTT (35μM) activated TRX-1 (1.75μM) was shown to be unaffected whether performed under argon or air; n = 2 (E-F).
Amplification of free thiol content within TRX-1–treated nβ2GPI by HUVECs. (A-B) nβ2GPI (1μM) was pretreated with DTT (35μM) activated TRX-1 (1.75μM) for 1 hour and then incubated with HUVECs or empty wells. (C-D) rβ2GPI (1μM) was pretreated with TRX-R (10nM)/NADPH (200μM) activated TRX-1 (1.75μM) for 1 hour and then incubated with HUVECs or empty wells. The supernatant from each well was then labeled with MPB, transferred to a PVDF membrane, and probed with streptavidin–horseradish peroxidase (HRP). This confirmed that HUVECs are also capable of amplifying the free thiol content of (A-B) nβ2GPI pretreated with DTT-activated TRX-1 (mean enhancement ± SD, 58.1% ± 32.5%; *P ≤ .04; n = 3) and (C-D) rβ2GPI pretreated with TRX-R/NADPH–activated TRX-1 (207.6% ± 146.4%; *P ≤ .03; n = 4). Efficiency of MPB labeling of DTT (35μM) activated TRX-1 (1.75μM) was shown to be unaffected whether performed under argon or air; n = 2 (E-F).
Although argon was necessary to maintain β2GPI in the reduced state after incubation with TRX-1, we show in Figure 3E and F that argon is not required for subsequent MPB association with free thiols.
Oxidoreductase proteins in addition to PDIs such as TRX-R and ERp 46 are secreted constitutively from endothelial cells
In the absence of TRX-1, purified β2GPI cannot be reduced directly by endothelial cells or by EAhy926 cells. Hence, the free thiol amplification effect by endothelial cells depends on the presence of TRX-1, implying that endothelial and EAhy926 cells amplify β2GPI MPB labeling through maintaining extracellular TRX-1 activity. Microarray studies were performed on unstimulated HUVECs to profile constitutive oxidoreductase transcript generation by these cells. All microarray data are available on the Gene Expression Omnibus (GEO) public database (National Center for Biotechnology Information [GEO DataSets]; http://www.ncbi.nlm.gov/sites/entrez) under accession number GSE22166.
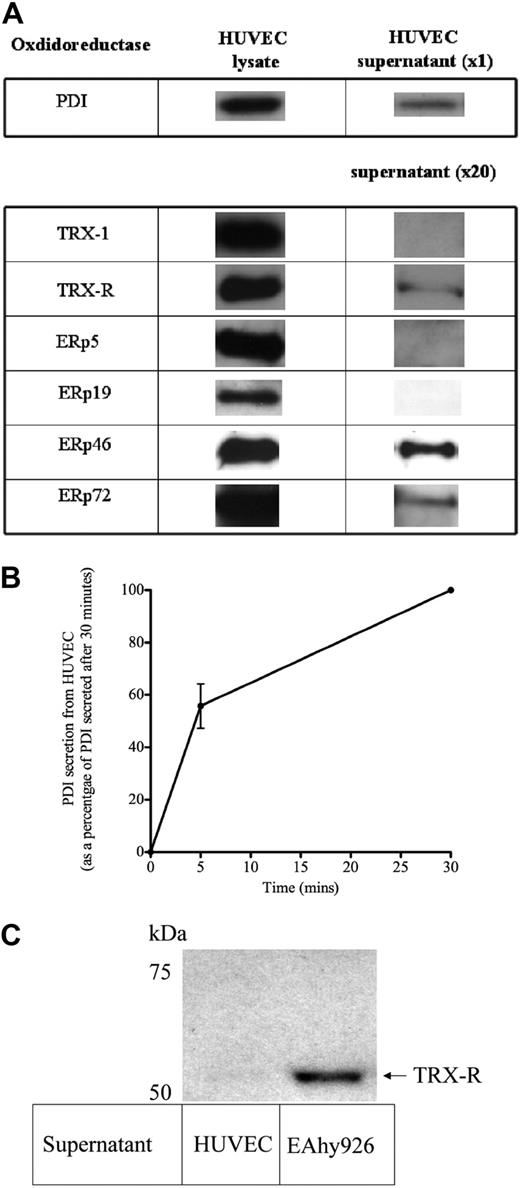
After analyses of the microarray data, isomerases of the TRX and PDI family seen at relatively high levels of signal intensity (ie, ≥ 10% β-actin and glyceraldehyde-3-phosphate dehydrogenase transcript levels) were PDI, TRX-1, TRX-R1 and TRX-R2, and the oxidoreductase PDI/TRX family of ERps, such as ERp46 (thioredoxin domain containing 5), ERp57 (PDIA3), ERp5 (PDIA6), and ERp72 (PDIA4; supplemental Table 1). Of these, ERp46 had the strongest signal intensity, equivalent to that seen with β-actin. Although these oxidoreductase enzymes are known to reside within the endoplastic reticulum, PDI as well as other oxidoreductase proteins such as ERp 57 and ERp 72 have also been detected within the plasma membrane wall of endothelial cells as described.34,35 Hence, we elected to screen for the panel of oxidoreductase enzymes identified from the microarray studies for evidence of secretion by endothelial cells. In addition to PDI and ERp 72, constitutive secretion of TRX-R and ERp46 in concentrated supernatant of unstimulated HUVECs was also shown (Figure 4A). ERp5 and TRX-1, although found in abundance in the lysate, were not detected in concentrated supernatant of unstimulated HUVECs. The immunoreactive band intensity of all the oxidoreductase enzymes studied was relatively equal in the lysate of cells (Figure 4A). However, only PDI could be detected in unconcentrated supernatant, indicating that PDI is the most abundantly secreted oxidoreductase. A time-course experiment showed that of the PDI constitutively secreted by HUVECs over 30 minutes, (55.7% ± 14.7%; n = 3) is secreted within the first 5 minutes (Figure 4B). Figure 4C shows that the supernatant of the human cell line EAhy926 also contains TRX-R. Interestingly, experiments done under identical conditions in parallel with HUVECs with the same cell number showed that TRX-R was detected in EAhy926 in unconcentrated supernatant, indicating a greater amount of TRX-R secretion for EAhy926 cells than for HUVECs. However, a relatively low concentration of TRX-R (10nM) was used to reduce TRX-1 (3.5μM). This concentration of TRX-R (10nM) is below the detection limit of the TRX-R immunoblot experiments used in this study (results not shown).
Multiple oxidoreductases secreted by unstimulated endothelial cells. (A) HUVECs grown to confluence on a 96-well plate, washed thoroughly, and then incubated with 30 μL/well of HBS buffer for 30 minutes at 37°C. HBS supernatant was then removed, and cells were lysed with a volume of lysis buffer equal to supernatant. Lysate neat (20 μL) or 20× concentrated HBS supernatant was then transferred to PVDF and probed for the relevant oxidoreductase. (B) HBS was incubated with HUVECs as above for 5 and 30 minutes, and neat supernatant was probed for PDI. Amount of PDI detected is expressed as arbitrary units. (C) HUVECs and EAhy926 cells were grown to confluence in parallel within the same 96-well plate, washed, and incubated with HBS buffer (30 μL/well) for 30 minutes. The buffer supernatant was then removed, and equal amounts of supernatant were transferred to PVDF membrane and probed with an anti–TRX-R antibody. A cell viability assay confirmed equivalent amounts of viable cells for HUVECs and EAhy926 cells. TRX-R was only detectable in HUVEC supernatant after concentration 20×, as shown in panel A. All blots are representative of 3 independent experiments.
Multiple oxidoreductases secreted by unstimulated endothelial cells. (A) HUVECs grown to confluence on a 96-well plate, washed thoroughly, and then incubated with 30 μL/well of HBS buffer for 30 minutes at 37°C. HBS supernatant was then removed, and cells were lysed with a volume of lysis buffer equal to supernatant. Lysate neat (20 μL) or 20× concentrated HBS supernatant was then transferred to PVDF and probed for the relevant oxidoreductase. (B) HBS was incubated with HUVECs as above for 5 and 30 minutes, and neat supernatant was probed for PDI. Amount of PDI detected is expressed as arbitrary units. (C) HUVECs and EAhy926 cells were grown to confluence in parallel within the same 96-well plate, washed, and incubated with HBS buffer (30 μL/well) for 30 minutes. The buffer supernatant was then removed, and equal amounts of supernatant were transferred to PVDF membrane and probed with an anti–TRX-R antibody. A cell viability assay confirmed equivalent amounts of viable cells for HUVECs and EAhy926 cells. TRX-R was only detectable in HUVEC supernatant after concentration 20×, as shown in panel A. All blots are representative of 3 independent experiments.
One or more free thiols generated within β2GPI by activated TRX-1 undergo S-nitrosylation
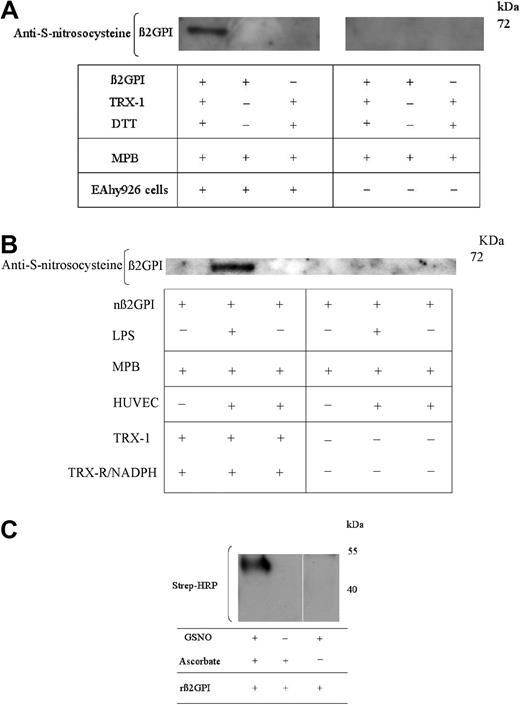
Given the potential for human endothelial and EAhy926 cells to modify the redox state of β2GPI, experiments were undertaken to assess whether free cysteine thiols generated within β2GPI had the potential for S-nitrosylation. nβ2GPI pretreated with TRX-1/DTT was incubated with unstimulated EAhy926 cells. The supernatant was labeled with MPB, transferred to a PVDF membrane, and probed with an anti–S-nitrosocysteine antibody, specific for S-nitrosylated cysteines. This identified an immunoreactive band at approximately 70 kDa, consistent with this being nitrosylated, reduced nβ2GPI (Figure 5A). In the absence of MPB, the anti-nitrosocysteine immunoreactivity was markedly reduced. Immunoreactivity was not observed both with non–TRX-1–treated nβ2GPI and in the absence of EAhy926 cells (Figure 5A). Interestingly, this experiment could not be repeated with unstimulated HUVECs; however, on prestimulating HUVECs with lipopolysaccharide, clear evidence of cell-mediated cysteine nitrosylation of reduced β2GPI was observed (Figure 5B).
One or more free thiols generated within TRX-1–treated β2GPI undergoes S-nitrosylation. (A) nβ2GPI (1μM) pretreated with DTT (20μM) activated TRX-1 was then incubated with EAhy926 cells for 20 minutes at 37°C. Equal amounts of supernatant were then labeled with MPB and probed for the presence of S-nitrosocysteines. S-nitrosocysteine formation within TRX-1–treated nβ2GPI is detected only after incubation with endothelial cells. (B) nβ2GPI (1μM) pretreated with TRX-R/NADPH–activated TRX-1 was incubated for 20 minutes at 37°C with unstimulated HUVECs, HUVECs pretreated with lipopolysaccharide (LPS; 100 ng/mL for 20 hours at 37°C) or with empty wells. S-nitrosocysteine formation was detected only in TRX-1–treated β2GPI and only after incubation with preactivated HUVECs. (C) rβ2GPI (1μM) pretreated with DTT-activated TRX-1 was then incubated with S-nitrosogluathione (GSNO). Free thiols were blocked with N-ethylmaleimide (NEM), and nitrosylated cysteine thiols then were exposed by degradation with ascorbic acid and labeled with MPB. Only TRX-1–treated rβ2GPI was treated with GSNO and ascorbate label with MPB. The above data are representative of 3 independent experiments for all panels.
One or more free thiols generated within TRX-1–treated β2GPI undergoes S-nitrosylation. (A) nβ2GPI (1μM) pretreated with DTT (20μM) activated TRX-1 was then incubated with EAhy926 cells for 20 minutes at 37°C. Equal amounts of supernatant were then labeled with MPB and probed for the presence of S-nitrosocysteines. S-nitrosocysteine formation within TRX-1–treated nβ2GPI is detected only after incubation with endothelial cells. (B) nβ2GPI (1μM) pretreated with TRX-R/NADPH–activated TRX-1 was incubated for 20 minutes at 37°C with unstimulated HUVECs, HUVECs pretreated with lipopolysaccharide (LPS; 100 ng/mL for 20 hours at 37°C) or with empty wells. S-nitrosocysteine formation was detected only in TRX-1–treated β2GPI and only after incubation with preactivated HUVECs. (C) rβ2GPI (1μM) pretreated with DTT-activated TRX-1 was then incubated with S-nitrosogluathione (GSNO). Free thiols were blocked with N-ethylmaleimide (NEM), and nitrosylated cysteine thiols then were exposed by degradation with ascorbic acid and labeled with MPB. Only TRX-1–treated rβ2GPI was treated with GSNO and ascorbate label with MPB. The above data are representative of 3 independent experiments for all panels.
Human β2GPI treated with activated TRX-1 protects endothelial and EAhy926 cell injury induced by oxidative stress
Intracellular TRX-1 acts as a powerful redox regulator,1 a property dependent not only on the generation of free thiols within the redox active center between Cys32 and Cys35 but also on S-nitrosylation of the free thiol located within the unpaired Cys69.2 Given the novel findings described thus far, the question was asked whether β2GPI could act as an extracellular regulator of oxidative stress–induced cell injury.
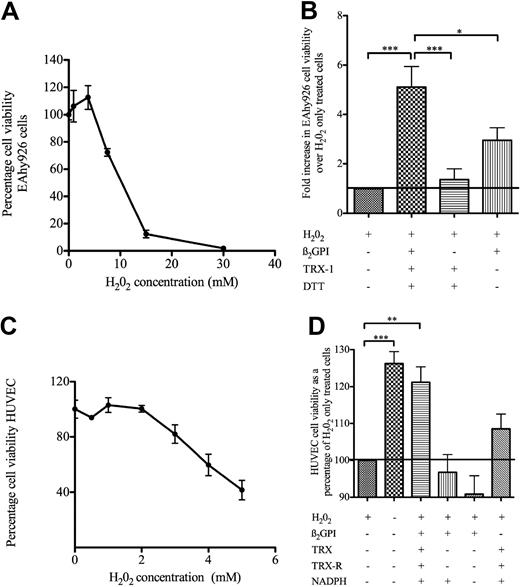
EAhy926 cells proved to be prone to H2O2-induced cell death at relatively high concentrations of H2O2. EAhy926 cells incubated with 7.5mM H2O2 for 20 minutes reduced cell viability from 100% plus or minus 3.8% (n = 6) to 72.22% plus or minus 2.8%, and 15mM H2O2 resulted in a drop of cell viability to 12.45% plus or minus 2.7% (Figure 6A). Thus, for experiments looking for protection from H2O2-induced cell death, 13mM H2O2 was used. Figure 6B shows that pretreating EAhy926 cells with nβ2GPI (1μM; pretreated with TRX-1/DTT) for 20 minutes and then with H2O2 for 20 minutes at 37°C resulted in marked protection of H2O2-induced cell death compared with H2O2-only–treated cells. No significant protection in H2O2-induced cell death was observed with nβ2GPI- or TRX-1/DTT–treated cells.
β2GPI pretreated with TRX-1 protects human endothelial cells and EAhy 926 cells from oxidative stress–induced cell death. (A) EAhy926 cells were exposed to various concentrations of H2O2 for 20 minutes at 37°C, and a cell viability dose-response curve was constructed (n = 3). (B) Cells were incubated with nβ2GPI (2 μM) with or without pretreatment with DTT-activated TRX-1 (3.5μM) or with TRX-1 alone for 20 minutes at 37°C, then with 13mM H2O2 for 20 minutes at 37°C. After overnight incubation with media, cell viability was determined and expressed as fold increase in cell viability over H2O2-only–treated cells (n ≥ 5). (C) HUVECs were exposed to various concentrations of H2O2 for 40 minutes at 37°C, and a cell viability dose-response curve was constructed (n = 5). (D) HUVECs incubated with nβ2GPI (1μM) pretreated with TRX-1 (1.75μM) were activated by TRX-R/NADPH for 20 minutes at 37°C and then incubated with 4mM H2O2 for 40 minutes at 37°C. No difference between H2O2-only–treated cells and β2GPI/NADPH, β2GPI alone, and TRX-1/TRX-R/NADPH alone was observed (n = 3). For all panels, ***P < .001, **P ≤ .01, and *P ≤ .05.
β2GPI pretreated with TRX-1 protects human endothelial cells and EAhy 926 cells from oxidative stress–induced cell death. (A) EAhy926 cells were exposed to various concentrations of H2O2 for 20 minutes at 37°C, and a cell viability dose-response curve was constructed (n = 3). (B) Cells were incubated with nβ2GPI (2 μM) with or without pretreatment with DTT-activated TRX-1 (3.5μM) or with TRX-1 alone for 20 minutes at 37°C, then with 13mM H2O2 for 20 minutes at 37°C. After overnight incubation with media, cell viability was determined and expressed as fold increase in cell viability over H2O2-only–treated cells (n ≥ 5). (C) HUVECs were exposed to various concentrations of H2O2 for 40 minutes at 37°C, and a cell viability dose-response curve was constructed (n = 5). (D) HUVECs incubated with nβ2GPI (1μM) pretreated with TRX-1 (1.75μM) were activated by TRX-R/NADPH for 20 minutes at 37°C and then incubated with 4mM H2O2 for 40 minutes at 37°C. No difference between H2O2-only–treated cells and β2GPI/NADPH, β2GPI alone, and TRX-1/TRX-R/NADPH alone was observed (n = 3). For all panels, ***P < .001, **P ≤ .01, and *P ≤ .05.
nβ2GPI reduced by the more physiologic TRX-R/NADPH–activated TRX-1 also protected against H2O2-induced HUVEC injury. HUVECs were much more susceptible to H2O2-induced cell injury than the EAhy926 cells, as reported by other groups.36 HUVECs grown on gelatin-coated wells and exposed to 4mM H2O2 for 40 minutes resulted in a decrease in cell viability from 100% plus or minus 14.5% to 59.7% plus or minus 22.0% (n = 5; Figure 6C). This reduction in cell viability was completely abrogated by preincubating cells with nβ2GPI pretreated with TRX-R/NADPH–activated TRX-1, increasing cell viability to the level of that seen with cells incubated with media alone (n = 3; P < .001; Figure 6D). No abrogation in H2O2-induced cell death was observed when preincubating HUVECs with nβ2GPI or TRX-1/TRX-R/NADPH.
Discussion
There are several novel findings that are described in this report.
(1) Free thiols occur naturally within circulating human and murine β2GPI and may be detected in both serum and plasma preparations.
(2) The process of free thiol generation within β2GPI by TRX-1 (activated by both DTT and TRX-R/NADPH) may be amplified in the presence of endothelial cells. The robustness of this result is underlined because this is shown with both native and recombinant β2GPI and also with both primary HUVECs and the EAhy926 human cell line, which is derived from HUVECs fused with a cancer cell line.
(3) Confirmation of recent reports35 that endothelial cells secrete multiple oxidoreductases and show for the first time that TRX-R and ERp 46 may be added to the list of secreted oxidoreductases.
(4) TRX-1–treated β2GPI undergoes cysteine nitrosylation and thus may be added to the growing lists of proteins that are known thus far to have the potential for S-nitrosylation.37 This is shown directly with an anti–S-nitrosocysteine antibody and indirectly with a method based on the established biotin switch technique.31
(5) The functional significance of free thiol–containing β2GPI may be to protect endothelial cells from oxidative stress–induced cell injury. This was shown with the use of β2GPI with free thiols generated through treatment with TRX-1/DTT and TRX-R/NADPH activated TRX-1 and with both primary HUVECs and the EAhy926 human cell line.
The constitutive secretion of PDI by endothelial cells has been reported by other groups,34 as has the secretion of multiple thiol isomerase enzymes by platelets.38 However, endothelial cell secretion of members of the thiol isomerase group other than PDI, namely ERp 57 and ERp 72, has only been described.35 We show for the first time evidence for HUVEC secretion of TRX-R and ERp46. Although platelets have been shown to secrete ERp 5,39 this was not detected in the supernatant of HUVECs, although it was abundantly present in the lysate. One hypothesis generated from these findings is that the presence of TRX-R on the endothelial surface may facilitate the maintenance of TRX-1 activation, thus amplifying free thiols within β2GPI. Although the concentration of TRX-1 found in serum is less than that used within this report, the concentration secreted at the cell surface is unknown and is probably to be much higher. Because TRX-1 is known to be secreted during conditions of high oxidative stress by cells such as platelets, this hypothesis may represent a novel mechanism for the regulation of endothelial cell injury during oxidative stress. This is underlined by the finding that only β2GPI pretreated with activated TRX-1 had a protective effect against oxidative stress–induced cell injury. Future gene silencing and functional blocking assay studies will help to determine which is the dominant constitutively secreted oxidoreductase that promotes this mechanism, potentially opening the door to new therapeutic targets for conditions related to oxidative stress–induced tissue injury.
Although no TRX-1 secretion was detected within unstimulated HUVECs in our experiments, activated platelets have been shown to secrete TRX-1.7 Furthermore, we have shown that other oxidoreductases such as PDI, which is secreted by both HUVECs and platelets, can also generate the free thiols within β2GPI.29 Hence, one may speculate that in vivo, activated platelets at the site of vascular injury and in close proximity to the endothelial surface may secrete oxidoreductases such as TRX-1 and PDI. Such proteins may interact with oxidoreductases secreted by endothelial cells, serving not only to modulate thrombus development40 but also to regulate the redox status of the microenvironment and to maintain proteins such as β2GPI in the reduced state, which we have shown serves a protective role in maintaining endothelial cell viability under conditions of oxidative stress. EAhy926 cells secreted more TRX-R than primary HUVECs, probably reflecting that this cell line is due to a hybrid fusion of HUVECs and A549 carcinoma cells. Neoplastic cells have been shown to secrete TRX-R,41 and it may in part account for why the EAhy926 cells were more resistant to oxidative stress–induced cell injury.
The formation of S-nitrosocysteines within β2GPI was confirmed directly with the use of an anti–S-nitrosocysteine antibody and indirectly with the use of a method based on the biotin switch protocol used by multiple groups.31,42 Although nitrosylation of reduced β2GPI was shown consistently with unstimulated EAhy926 cells, unstimulated HUVECs did not show the same effect. However, prestimulating HUVECs with lipopolysaccharide resulted in cell-mediated nitrosylation of free cysteine thiols within TRX-1–treated β2GPI. Biochemical characteristics that predict susceptibility to nitrosylation of free thiol cysteines are (1) location within a hydrophobic region of the protein and (2) being flanked in close proximity by basic and acidic residues within the tertiary structure.43 On analyzing the crystal structure of β2GPI,25,26 these biochemical properties are fulfilled by Cys288 in the C-terminal domain, which has less than 5% surface exposure and resides adjacent to a hydrophobic loop. Our group has shown with the use of mass spectrometry that Cys326 represents the dominant cysteine residue that contains free thiols after incubation with activated TRX-1.29 This cysteine within the oxidized purified protein forms a disulfide bond with Cys288. Hence, once this bond is cleaved on incubation with TRX-1, Cys288 would have a free thiol available for nitrosylation.
Note that the effect of TRX-1–treated β2GPI was to protect endothelial cells and EAhy926 cells against oxidative stress, yet the function on platelets is one of promoting platelet adhesion to von Willebrand factor.44 Although this may seem paradoxical, other proteins such as PDI have also been described as having both procoagulant effects on platelets and protective effects on endothelial cells.40,45,46 Furthermore, during periods of coagulation, the maintenance of vascular endothelial integrity may be important in supporting crucial chemical and cellular interactions, and reduced β2GPI may have a dual role in promoting such mechanisms.
Finally, the relevance of the findings described here is underlined by showing, for the first time, that both human and murine β2GPI contain free cysteine thiols that may be labeled with MPB. The process of purification of β2GPI most probably results in oxidation of the cysteines, because purified β2GPI could not be labeled with MPB. It is of importance to determine the approximate proportion of β2GPI circulating in vivo in the reduced form, because this has potential implications in the relevance of studies of β2GPI biology with the use of the purified protein. One other possibility not excluded could be that purification of β2GPI selectively purifies oxidized β2GPI but not the free thiol–containing protein. MPB labeling of β2GPI within serum ex vivo was also shown with the use of a murine β2GPI knockout mouse model as the ideal negative control. The unique and relatively simple ELISA protocol that has been developed to detect β2GPI with free thiols allows for the screening of a wide variety of patient samples to ascertain the relevance of redox-modified β2GPI in vivo in conditions in which oxidative stress is pertinent to their pathogenesis. This ELISA even has the potential to be applied to the detection of other free thiol–containing extracellular proteins and to determine how levels may alter in disease. Future studies such as these and ongoing work aimed at delineating the underlying mechanism through which cell-surface oxidoreductases interact with substrate proteins such as β2GPI may show novel pathways of vascular wall pathology during oxidative stress, facilitating the identification of novel targets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
We thank Dr C. J. S. Edgell (University of North Carolina, Chapel Hill) for providing the human endothelial cell line EAhy926.
This work was supported by Arthritis Research UK through a Clinician Scientist Fellowship (grant 17821; Y.I.), by a grant from the Foundation of the Greek Society of Hematology (F.H.P.), by a National Health and Medical Research Council (NH&MRC) Postgraduate Scholarship (B.G.), and by project grants from NH&MRC.
Authorship
Contribution: Y.I., J.-Y.Z., B.G., and S.A.K. contributed to the experimental design; Y.I., J.-Y.Z., S.R., J.C.Q., B.G., M.Q., and P.Y. collected data; D.M.Y. and S.A.K. supervised the study; Y.I., F.H.P., P.J.H., B.G., and S.A.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven A. Krilis, Department of Immunology, Allergy, and Infectious Diseases, St George Hospital, University of New South Wales, 2 South St, Kogarah, 2217 Sydney, NSW, Australia; e-mail: s.krilis@unsw.edu.au.
References
Author notes
Y.I. and J.-Y.Z. contributed equally to this study.