Abstract
A key event in the successful induction of adaptive immune responses is the antigen-specific activation of T cells by dendritic cells (DCs). Although LFA-1 (lymphocyte function–associated antigen 1) on T cells is considered to be important for antigen-specific T-cell activation, the role for LFA-1 on DCs remains elusive. Using 2 different approaches to activate LFA-1 on DCs, either by deletion of the αL-integrin cytoplasmic GFFKR sequence or by silencing cytohesin-1–interacting protein, we now provide evidence that DCs are able to make use of active LFA-1 and can thereby control the contact duration with naive T cells. Enhanced duration of DC/T-cell interaction correlates inversely with antigen-specific T-cell proliferation, generation of T-helper 1 cells, and immune responses leading to delayed-type hypersensitivity. We could revert normal interaction time and T-cell proliferation to wild-type levels by inhibition of active LFA-1 on DCs. Our data further suggest that cytohesin-1–interacting protein might be responsible for controlling LFA-1 deactivation on mature DCs. In summary, our findings indicate that LFA-1 on DCs needs to be in an inactive state to ensure optimal T-cell activation and suggest that regulation of LFA-1 activity allows DCs to actively control antigen-driven T-cell proliferation and effective immune responses.
Introduction
The successful induction of an adaptive immune response is initiated through the antigen-specific activation of T cells by professional antigen-presenting cells (APCs) such as dendritic cells (DCs). Immature DCs take up antigens in the periphery, subsequently mature, and migrate to lymphoid organs, where they present antigens to naive T cells. Multiphoton microscopy studies in lymph node indicate that the interaction of naive T cells with DCs occurs in different phases: (1) scanning of different antigen-major histocompatibility complexes (MHCs) by T cells during short-lived DC/T-cell contacts, (2) formation of stable DC/T-cell contacts on MHC-peptide engagement, and (3) proliferation and detachment from the DC that allows activated T cells to migrate to sites of inflammation.1-4 A tight regulation of DC/T-cell adhesion during these heterogeneous contacts is fundamental. High-affinity lymphocyte function–associated antigen 1 (LFA-1) on T cells has been demonstrated to stabilize their interaction with DCs and is believed to act as a costimulator for T-cell activation.5 Observations that demonstrated that the maturation state of DCs can control T-cell/DC contact duration6 and the fact that DCs regulate intercellular adhesion molecule-1 (ICAM-1) to a maximum level exactly when T cells stably arrest on the DC surface7 led to the speculation that cell adhesion molecules on DCs may actively control antigen-specific T-cell activation.
Although the β2-integrins LFA-1 and macrophage-1 antigen (Mac-1) are expressed on DCs, their function during antigen-specific T-cell activation is only poorly understood. In the absence of β2-integrins on DCs, normal T-cell activation has been observed.8 Moreover, activation of β2-integrins with divalent cations resulted in decreased allogen-specific T-cell proliferation that was dependent on Mac-1.8 Thus, active Mac-1 on DCs might even be inhibitory for T-cell function. The role of LFA-1 in this context is less clear. Electron microscopy studies have indicated that LFA-1 on DCs is expressed in a random distribution of single inactive molecules excluded from lipid rafts.9 Even in the presence of integrin-activating stimuli, LFA-1 binding of mature DCs to ICAM-1 could not be induced,8,9 which suggests that LFA-1 on DCs is expressed in an inactive state. Furthermore, inhibition of LFA-1 on mature DCs before their first T-cell contact appears to have no influence on antigen-specific T-cell proliferation,10 which indicates that at least on mature DCs, LFA-1 might be dispensable during the formation of initial T-cell/DC contacts.
Resting leukocytes express integrins in an inactive state. Integrin adhesion can be induced both by clustering proteins on the membrane surface to increase the number of integrin interactions and by conformational changes in the integrin extracellular domain of individual proteins to increase their affinity for ligands.11 DCs have been demonstrated to express cytohesin-1, a molecule known to interact with the cytoplasmic β-subunit of LFA-1,12 thereby increasing LFA-1 affinity.13,14 Association of the cytohesin-1–interacting protein (CYTIP) relocalizes cytohesin-1 from the plasma membrane to the cytosol15 and leaves LFA-1 in a low-affinity state on the DC surface. On maturation of DCs, expression of CYTIP is up-regulated,15 which suggests that high levels of CYTIP prevent LFA-1 activation on mature DCs.
Because LFA-1 is clearly present on immature and mature DCs, the question remains whether LFA-1 must be kept in an inactive state for full antigen-specific T-cell activation. Using 2 different approaches that interfere with LFA-1 activity, we examined the role of deactivating LFA-1 on mature DCs for T-cell activation. Absence of the αL-integrin cytoplasmic GFFKR sequence is known to constitutively activate LFA-1 on T cells in vitro.16 By deleting the cytoplasmic GFFKR motif of αL-integrin in mouse germline (LFA-1d/d), we found adhesion of LFA-1d/d T lymphocytes to endothelial ICAM-1 to be markedly induced17 and efficient antigen-specific T-cell proliferation to be impaired.17,18
We now provide evidence that forcing expression of active LFA-1 allows mature DCs to adhere to ICAM-1. Activation of LFA-1 on DCs either by the LFA-1d/d mutation or by silencing of CYTIP expression induces the formation of prolonged contacts between antigen-loaded DCs and naive CD4+ or CD8+ T cells. Enhanced duration of DC/T-cell interaction appears to be inversely correlated with T-cell activation and antigen-specific T-cell proliferation, and expression of active LFA-1 on DCs was inhibitory for effective in vivo immune responses, which led to a delayed-type hypersensitivity reaction. The present data reveal a new function for LFA-1 activity regulation on mature DCs and indicate that inactive LFA-1 might be required for full antigen-specific T-cell activation in vitro and in vivo.
Methods
Mice
C57BL/6 mice were obtained from Harlan. T-cell receptor (TCR) transgenic OT-I mice and OT-II mice19 were kindly provided by T. Blankenstein (Max Delbrück Center, Berlin, Germany) and A. Reske-Kunz (Dermatology Clinic of the Johannes Gutenberg University of Medicine, Mainz, Germany). LFA-1d/d mice17 and CD18−/− mice20 have been described previously. Mice were housed under specific pathogen-free conditions, and all mice experiments were approved by the regional animal care committees.
Generation and culture of bone marrow–derived DCs
DCs were generated as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Fluorescence-activated cell sorter analysis
Expression of DC surface molecules was performed with the following monoclonal antibodies: αCD11c-allophycocyanin (HL-3), αCD86-FITC (fluorescein isothiocyanate; Gl-1), αCD80-PE (phycoerythrin; 16-10A1), αCD40-PE (3/23), αCD54-FITC (3E2), αCD18-PE (M18/2), αCD11a-FITC (M17/4), and αIA/IE-FITC (2G9), all obtained from BD Biosciences, and analyzed with the FACSCanto or LSR-II with Diva software Version 6.1.1 (BD Biosciences). Interferon-γ (IFN-γ) expression in CD4+ OT-II T cells 24 hours after coculture with antigen-loaded DCs was quantified by intracellular fluorescence-activated cell sorter staining with BD Cytofix/Cytoperm Plus, a Golgi Plus kit, and αIFN-γ-allophycocyanin (XMG1.2) plus monoclonal antibodies against αCD4-FITC (RM4-5) and αCD11c-PE (N418), all from BD Biosciences.
Antibody treatment of DCs
Mature DCs were harvested and preincubated with 10 μg/mL of function-blocking αCD18 antibody (GAME46; BD Biosciences), αCD11a antibody (FD441.8; Leinco Technologies via Biotrend), and nonblocking αCD18 antibody (M18/2) or rat immunoglobulin G2a isotype control antibody (BD Biosciences) for 2 hours. If mentioned, DCs were additionally loaded with ovalbumin peptides (OVA or SIINFEKL) during the last 60 minutes. Afterward, cells were washed twice and used in functional assays.
T-lymphocyte isolation
Untouched naive splenic CD8+ T cells of OT-I mice (SIINFEKL specific) and untouched naive splenic CD4+ T cells of OT-II mice (OVA specific) were enriched to a purity of > 96% by negative selection with the RoboSep CD8+ and CD4+ T-cell kit (StemCell Technologies), respectively. Purified CD8+ or CD4+ T cells were cultured in RPMI supplemented with 5% fetal calf serum, 1× nonessential amino acids, 2mM l-glutamine, 10mM HEPES, 1mM sodium pyruvate, 500nM 2-ME, and 100 U/mL penicillin/streptomycin. The purity of isolated T cells was controlled by flow cytometry with αCD8-FITC (53-6.7) or αCD4-FITC (RM4-5) and αCD3-PE (145.2C11) monoclonal antibodies (all from BD Biosciences).
ICAM-1 adhesion assay
Ninety-six–well plates were coated with 1.5 μg/mL recombinant mouse ICAM-1/Fc chimera or with human immunoglobulin G (both from R&D Systems) at 4°C for 24 hours. Assays were performed with 3.5 × 105 wild-type or LFA-1d/d DCs for 60 minutes at 37°C exactly as described previously.8 Numbers of adherent cells were evaluated by computer-aided image analysis with National Institutes of Health Image 1.55 software.
Cell-cell interactions within 3-dimensional collagen gels
Time-lapse video microscopy analysis of DC/T-cell interactions within 3-dimensional (3-D) collagen gels were performed as described previously.21 Briefly, DCs were loaded with 1 μg/mL ovalbumin peptides (aa 323-339, lipopolysaccharide free) or with 0.1 μg/mL SIINFEKL antigen (aa 257-264, lipopolysaccharide free; both from Neo MPS PolyPeptide Laboratories) and cocultivated with naive CD4+ OT-II and CD8+ OT-I T cells in 3-D collagen gels at 37°C, respectively. Contact (T-cell pausing or crawling) duration in minutes was monitored simultaneously by time-lapse microscopy with an Olympus BX61 microscope with a UApo lens (20 × 340, numerical aperture 0.75) and an F-View camera with CellP software (2006; Soft Imaging Systems). In some experiments, 24 hours after the coculture was begun, 3-D collagen matrices were digested by type III collagenase (30 U/100 mL gel; Sigma) for 30 minutes at 37°C, and T cells were analyzed by flow cytometry or quantitative real-time polymerase chain reaction (PCR).
Antigen-specific T-cell proliferation
Antigen-pulsed DCs were titrated in triplicate within 3-D collagen gels, and antigen-specific CD4+ OT-II or CD8+ OT-I T cells were added to obtain DC/T-cell ratios of 1:10, 1:30, 1:90, and 1:270. Alternatively, different antigen doses (0.01, 0.1, and 1 μg/mL SIINFEKL) were titrated onto DCs within 3-D collagen gels at a DC/T-cell ratio of 1:10. Cells were incubated for 3 days at 37°C and 5% CO2. Subsequently, cells were labeled with [3H]thymidine (37 000 Bq/well) for 16 hours, gels were digested with collagenase as described in the previous paragraph, and T-cell proliferation was assessed by incorporation of [3H]thymidine measured by liquid scintillation counting.
TCR down-regulation
DCs were loaded with different antigen doses (0.01, 0.1, and 1 μg/mL SIINFEKL) and cocultivated with freshly isolated CD8+ OT-I T cells within 3-D collagen gels at DC/T-cell ratios of 1:10 for 3 hours. After gel digestion by collagenase, T cells were stained with the monoclonal antibodies αCD3-PE-Cy5 (145-2C11; Biolegend), αCD8-allophycocyanin-Cy7 (53-6.7; BD Biosciences), and αVα2-allophycocyanin (B2.1; eBioscience). Down-regulation of Vα2 was analyzed with the fluorescence-activated cell sorter LSR-II with Diva software Version 6.1.1 (BD).
Silencing of CYTIP with small interfering RNA
Down-regulation of CYTIP expression by small interfering RNA (siRNA) transfection was performed as described in supplemental Methods.
Delayed-type hypersensitivity
A total of 5 × 106 OVA-specific CD4+ T cells from OT-II mice were transferred into syngeneic C57BL/6 mice. The next day, 5 × 105 syngeneic OVA antigen–loaded DCs from wild-type or LFA-1d/d mice were injected into hind footpads. After an incubation period of 17 days, mice were injected a second time with 5 × 105 OVA peptide–loaded wild-type or LFA-1d/d DCs, respectively. Twenty-four hours later, footpad swelling was measured for more a period of more than 8 days.
Quantitative Real-Time PCR
Interleukin-2 (IL-2), Tbet, GATA3, and FoxP3 mRNA in activated T cells were quantified as described in supplemental Methods.
Statistical analysis
Statistical differences between mean values were calculated by the Student t test for unpaired data. P < .05 was considered statistically significant.
Results
Active LFA-1 induces DC adhesion to ICAM-1
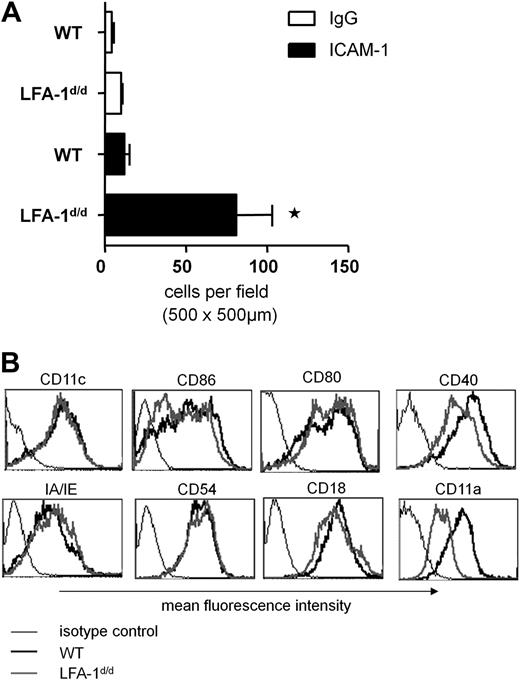
We previously found that locking LFA-1 in an active state induces adhesion of naive T cells to its ligand, ICAM-1.17 In contrast to T cells, β2-integrins on DCs appear to play only a minor role in mediating cell adhesion.8,9 To test whether DCs are able to use active LFA-1 to bind ICAM-1 in principle, we performed a static adhesion assay on recombinant ICAM-1/Fc. All studies were performed with granulocyte-macrophage colony-stimulating factor–treated bone marrow–derived DCs that were stimulated with anti-CD40 and thus were representative of mature DCs. Although wild-type DCs adhered to ICAM-1 only to a low extent, expression of constitutively active LFA-1 on LFA-1d/d DCs significantly increased adhesion to ICAM-1 (Figure 1A); however, expression of surface markers typical for the maturation state of DCs was not significantly altered in LFA-1d/d DCs (Figure 1B; supplemental Figure 1). Consistent with previous observations regarding LFA-1d/d T cells,17 LFA-1 expression on the LFA-1d/d DC surface was reduced compared with wild-type LFA-1. Taken together, these results demonstrate that expression of active LFA-1 allows mature DCs to bind ICAM-1.
Active LFA-1 on mature DCs induces their adhesion to ICAM-1.(A) Mature bone marrow–derived DCs from wild-type (WT) or LFA-1d/d mice were incubated with recombinant ICAM-1/Fc or with control immunoglobulin G (IgG), and the number of adherent cells was evaluated. Mean of n = 3 experiments (each experiment was performed in duplicate). *P < .05. (B) Cell surface markers known to be induced during maturation of DCs were expressed to a similar extent in mature wild-type and LFA-1d/d DCs, as shown by flow cytometry (1 representative assay of 6). Surface expression of mutant compared with wild-type LFA-1 on DCs was reduced.
Active LFA-1 on mature DCs induces their adhesion to ICAM-1.(A) Mature bone marrow–derived DCs from wild-type (WT) or LFA-1d/d mice were incubated with recombinant ICAM-1/Fc or with control immunoglobulin G (IgG), and the number of adherent cells was evaluated. Mean of n = 3 experiments (each experiment was performed in duplicate). *P < .05. (B) Cell surface markers known to be induced during maturation of DCs were expressed to a similar extent in mature wild-type and LFA-1d/d DCs, as shown by flow cytometry (1 representative assay of 6). Surface expression of mutant compared with wild-type LFA-1 on DCs was reduced.
Prolonged interaction of LFA-1d/d DCs with naive T cells
A considerable long-lasting interaction between naive T cells and APCs is believed to be essential for antigen-specific T-cell activation. Yet, T-cell/APC interactions need to be transient to allow T cells to efficiently scan different MHC-peptide complexes on the surface of 1 or even several DCs. Because expression of active LFA-1 enables DC adhesion to ICAM-1, we raised the question of whether antigen-loaded DCs also use LFA-1 to attach to naive T cells. Therefore, ovalbumin peptide–pulsed DCs from wild-type or LFA-1d/d mice were cocultivated with naive TCR-transgenic T lymphocytes, and their interaction was visualized in a 3-D collagen matrix. We found the mean interaction time of CD4+ OT-II and CD8+ OT-I T cells with LFA-1d/d DCs was increased significantly (Figure 2A,C). The number of different T-cell/DC interactions could be subdivided into 2 groups: (1) T cells that stayed in contact with DCs for 5 to 25 minutes (short contacts) or (2) T cells that made contacts that lasted longer than 60 minutes (long-lived contacts). Surprisingly, we found no major influence of active LFA-1 on short DC/T-cell contacts, which suggests that T-cell scanning of DCs occurs independent of DC LFA-1. Quantifying the number of contacts that lasted longer than 60 minutes, we detected significantly more CD4+ and CD8+ T cells that interacted with LFA-1d/d than with wild-type DCs (Figure 2B,D). Induction of longer contacts resulted in an accumulation of multiple T cells on LFA-1d/d DCs (supplemental Figure 2). The present data indicated that active LFA-1 on DCs specifically affected the formation of long-lived contacts by strengthening their interaction with T cells.
Active LFA-1 on DCs enhances the formation of long-lived contacts with naive T cells. Ovalbumin peptide–loaded mature wild-type (WT) or LFA-1d/d DCs were cocultivated with untouched naive CD4+ OT-II (A) and CD8+ OT-I (C) T cells in 3-D collagen. Contact times of individual T-cell/DC pairs were analyzed during the first 12 hours of coculture. Numbers of contacts between DCs and CD4+ (B) and CD8+ (D) T cells that lasted longer than 60 minutes were counted.
Active LFA-1 on DCs enhances the formation of long-lived contacts with naive T cells. Ovalbumin peptide–loaded mature wild-type (WT) or LFA-1d/d DCs were cocultivated with untouched naive CD4+ OT-II (A) and CD8+ OT-I (C) T cells in 3-D collagen. Contact times of individual T-cell/DC pairs were analyzed during the first 12 hours of coculture. Numbers of contacts between DCs and CD4+ (B) and CD8+ (D) T cells that lasted longer than 60 minutes were counted.
Inactive LFA-1 on DCs is critical for T-cell activation and proliferation
The formation of long-lived contacts has been demonstrated to be critical for productive DC/T-cell interactions that result in antigen-specific T-cell proliferation.22 Because active LFA-1 on DCs positively regulates their engagement with T cells, we investigated whether constitutively active LFA-1 (LFA-1d/d) also positively influences the proliferation of naive TCR-transgenic T cells cocultivated with antigen-pulsed DCs. Surprisingly, we found the proliferation of CD4+ OT-II and CD8+ OT-I T cells induced by LFA-1d/d DCs to be reduced compared with wild-type DCs (Figure 3A,B), which demonstrates that active LFA-1 on DCs negatively influences antigen-specific proliferation of CD4+ and CD8+T cells. Internalization of the TCR on TCR/peptide-MHC ligation is known to be an early event of antigen-specific T-cell activation.23 The ability of different peptide doses to cause endocytosis of the TCR on CD8+ OT-I T cells cocultivated with wild-type DCs correlated well with their ability to induce T-cell proliferation (Figure 4A,B). The amount of TCR down-regulation induced by wild-type and LFA-1d/d DCs did not differ significantly (Figure 4A), which indicates that recognition of peptide-MHC complexes was not disturbed by the presence of active LFA-1 on the DC surface.
Active LFA-1 on DCs negatively influences T-cell proliferation. Mature DCs from wild-type (WT) and LFA-1d/d mice were loaded with ovalbumin peptides and cocultivated in different concentrations with naive CD4+ OT-II (A) and CD8+ OT-I (B) in a 3-D collagen gel for 3 days. Cell proliferation was assessed by incorporation of [3H]thymidine. Assays were performed in triplicate. One representative assay of 3 is shown. cpm indicates counts per minute.
Active LFA-1 on DCs negatively influences T-cell proliferation. Mature DCs from wild-type (WT) and LFA-1d/d mice were loaded with ovalbumin peptides and cocultivated in different concentrations with naive CD4+ OT-II (A) and CD8+ OT-I (B) in a 3-D collagen gel for 3 days. Cell proliferation was assessed by incorporation of [3H]thymidine. Assays were performed in triplicate. One representative assay of 3 is shown. cpm indicates counts per minute.
Active LFA-1 on DCs interferes with T-cell activation and generation of Th1 cells. (A) OT-I T cells were cocultivated for 4 hours with wild-type (WT) or LFA-1d/d DCs loaded with different doses of the ovalbumin peptide SIINFEKL in a 3-D collagen matrix. On isolation from the matrix, expression of the Vα2-chain of the TCR was measured by flow cytometry. Mean fluorescence intensity (MFI) of Vα2 on CD8+ T cells is depicted (n = 3). Amount of TCR down-regulation by wild-type or LFA-1d/d DCs was found to be insignificant. (B) OT-I T cells were activated by wild-type or LFA-1d/d DCs pulsed with different doses of SIINFEKL for 3 days in parallel to experiments performed in panel A. Proliferation was measured by incorporation of [3H]thymidine (n = 2). (C) OT-II T cells and ovalbumin peptide–loaded DCs were isolated from the collagen matrix after 24 hours of coculture. Total RNA was extracted, and quantitative real-time PCR was performed. RNA expression levels were normalized to expression of endogenous β-actin mRNA. Relative expression of IL-2 mRNA of wild-type DCs cocultivated with OT-II T cells in the absence of peptide was equalized to 1 (n = 3). *P < .05. (D) Naive CD4+ OT-II cells were cocultivated for 24 hours with DCs in the presence or absence of ovalbumin peptide in a 3-D collagen matrix. Expression of intracellular IFN-γ was analyzed by flow cytometry. The number of IFN-γ-positive CD4+ T cells was quantified (n = 2). *P < .05. (E) Total RNA from CD4+ OT-II T cells cocultivated with ovalbumin-loaded DCs in a 3-D collagen gel for 24 hours was extracted, and quantitative real-time PCR was performed. RNA expression levels were normalized to expression of endogenous β-actin mRNA. Relative expression of Tbet (n = 4), GATA3 (n = 4), and FoxP3 (n = 3) mRNA of wild-type DCs cocultivated with OT-II T cells was equalized to 1. *P < .05. cpm indicates counts per minute.
Active LFA-1 on DCs interferes with T-cell activation and generation of Th1 cells. (A) OT-I T cells were cocultivated for 4 hours with wild-type (WT) or LFA-1d/d DCs loaded with different doses of the ovalbumin peptide SIINFEKL in a 3-D collagen matrix. On isolation from the matrix, expression of the Vα2-chain of the TCR was measured by flow cytometry. Mean fluorescence intensity (MFI) of Vα2 on CD8+ T cells is depicted (n = 3). Amount of TCR down-regulation by wild-type or LFA-1d/d DCs was found to be insignificant. (B) OT-I T cells were activated by wild-type or LFA-1d/d DCs pulsed with different doses of SIINFEKL for 3 days in parallel to experiments performed in panel A. Proliferation was measured by incorporation of [3H]thymidine (n = 2). (C) OT-II T cells and ovalbumin peptide–loaded DCs were isolated from the collagen matrix after 24 hours of coculture. Total RNA was extracted, and quantitative real-time PCR was performed. RNA expression levels were normalized to expression of endogenous β-actin mRNA. Relative expression of IL-2 mRNA of wild-type DCs cocultivated with OT-II T cells in the absence of peptide was equalized to 1 (n = 3). *P < .05. (D) Naive CD4+ OT-II cells were cocultivated for 24 hours with DCs in the presence or absence of ovalbumin peptide in a 3-D collagen matrix. Expression of intracellular IFN-γ was analyzed by flow cytometry. The number of IFN-γ-positive CD4+ T cells was quantified (n = 2). *P < .05. (E) Total RNA from CD4+ OT-II T cells cocultivated with ovalbumin-loaded DCs in a 3-D collagen gel for 24 hours was extracted, and quantitative real-time PCR was performed. RNA expression levels were normalized to expression of endogenous β-actin mRNA. Relative expression of Tbet (n = 4), GATA3 (n = 4), and FoxP3 (n = 3) mRNA of wild-type DCs cocultivated with OT-II T cells was equalized to 1. *P < .05. cpm indicates counts per minute.
In addition to these early signals, successful T-cell proliferation requires sustained TCR engagement to allow activation of multiple signaling pathways that regulate later events of T-cell activation, such as cell division and cytokine production. Consistent with impaired T-cell proliferation, mRNA levels of IL-2 were significantly reduced in CD4+ T cells cocultivated with LFA-1d/d DCs compared with T cells activated by wild-type DCs (Figure 4C). In support of these results, the number of IFN-γ–positive T cells was found to be reduced to background levels when T cells were cocultivated with LFA-1d/d DCs, whereas antigen-loaded wild-type DCs induced intracellular IFN-γ expression by T cells (Figure 4D). Thus, the present data suggest that active LFA-1 on DCs not only impairs T-cell activation and proliferation but might also influence the generation of IL-2/IFN-γ–producing effector T-helper (Th) 1 cells. To test this hypothesis, we examined Th1-, Th2-, or regulatory T-cell–driven mRNA expression of Tbet, GATA3, and FoxP3 in CD4+ T cells cocultivated with antigen-loaded wild-type or LFA-1d/d DCs. Although mRNA levels of GATA3 and FoxP3 in CD4+ T cells did not appear to be affected by the expression of active LFA-1 on DCs, a significantly reduced amount of Tbet mRNA was detected when T cells were cocultivated with LFA-1d/d DCs (Figure 4E). Taken together, we provide evidence that the prevention of LFA-1 inactivation on DCs enhances DC/T-cell interaction and impairs antigen-specific T-cell proliferation and generation of Th1 effector cells.
Expression of active LFA-1 on DCs reduces delayed-type hypersensitivity reaction
To corroborate our in vitro results, we analyzed in vivo inflammatory responses in a delayed-type hypersensitivity reaction considered to be driven by activated Th1 cells. Adoptive transfer of naive CD4+ OT-II T cells followed by a 2-fold subcutaneous injection of antigen-loaded wild-type DCs induced footpad swelling, which reached a maximum 2 days after final DC transfer (Figure 5). Expression of active LFA-1 on LFA-1d/d DCs resulted in reduced cutaneous swelling of hind footpads by 47% at day 2 after DC transfer (Figure 5). Although footpad swelling declined at later time points, diminished skin thickening could be observed during the entire observation period when LFA-1d/d DCs were applied to wild-type recipients. Given these results, we suggest that regulation of LFA-1 activity on DCs might be responsible for effective immune responses by controlling activation and proliferation of antigen-specific T cells in vivo.
Expression of active LFA-1 on DCs attenuates delayed-type hypersensitivity response. Ovalbumin-specific naive CD4+ OT-II T cells were transferred into wild-type (WT) syngeneic recipients, followed by a 2-fold injection of ovalbumin peptide–pulsed wild-type or LFA-1d/d DCs into hind footpad 24 hours and 17 days after T-cell transfer. Twenty-four hours after the final DC transfer, footpad swelling was measured (n = 8 mice). *P < .05, **P < .01, #P < .001.
Expression of active LFA-1 on DCs attenuates delayed-type hypersensitivity response. Ovalbumin-specific naive CD4+ OT-II T cells were transferred into wild-type (WT) syngeneic recipients, followed by a 2-fold injection of ovalbumin peptide–pulsed wild-type or LFA-1d/d DCs into hind footpad 24 hours and 17 days after T-cell transfer. Twenty-four hours after the final DC transfer, footpad swelling was measured (n = 8 mice). *P < .05, **P < .01, #P < .001.
Loss of CYTIP expression in DCs induces longer contacts with T cells and negatively interferes with T-cell proliferation
CYTIP has been implicated in the control of β2-integrin deactivation on DCs via recruitment of cytohesin-1 from the plasma membrane to the cytoplasm.15 CYTIP knockdown in DCs has been demonstrated to impair antigen-specific CD8+ T-cell expansion.24 However, it has not been explored whether changes in DC/T-cell contact time or changes in LFA-1 activity on DCs might be responsible for this observation. To address the question of contact time, we silenced CYTIP in wild-type DCs and visualized their interaction with naive T cells in a 3-D collagen matrix. Mature DCs left untreated or transfected with a control siRNA expressed CYTIP mainly in the cytoplasm (Figure 6A; supplemental Figure 3). Transfection of DCs with CYTIP-specific siRNA significantly reduced CYTIP expression in the majority of cells (Figure 6A) but did not affect expression of activation-dependent surface molecules (Figure 6B), which confirms data by Hofer and colleagues.24
CYTIP silencing in DCs induces prolonged contacts with naive T cells and impairs antigen-specific T-cell proliferation. (A) Knockdown of CYTIP expression was monitored by immunofluorescence staining of mature DCs 24 hours after transfection with unrelated control and CYTIP siRNA, respectively. Original magnification ×63. (B) Expression of surface markers known to be up-regulated on maturation of DCs was analyzed by flow cytometry after transfection of wild-type DCs with control siRNA and specific CYTIP siRNA, respectively. Surface staining of CD80 or CD40 on CD11c+ DCs is depicted in the 2 bottom rows and was found not to be significantly different in CYTIP-silenced versus control DCs. Specific staining for CD11c, CD80, and CD40 is indicated by filled lines; isotype control is illustrated by unfilled lines. The geometric mean of specific staining was quantified and is shown within each fluorescence-activated cell sorter profile. (C) Mature DCs were transfected with control or CYTIP-specific siRNA and cocultivated in presence of the ovalbumin peptide with naive CD4+ OT-II T cells in a 3-D collagen gel. Contact times of individual T-cell/DC pairs were analyzed and were found to be significantly enhanced on silencing of CYTIP expression. (D) Antigen-specific proliferation of CD4+ OT-II T cells on cocultivation with DCs that were left untreated or were transfected with control or CYTIP siRNA was assessed by incorporation of [3H]thymidine. Assays were performed in triplicate. One representative assay of 3 is shown. *P < .05. cpm indicates counts per minute.
CYTIP silencing in DCs induces prolonged contacts with naive T cells and impairs antigen-specific T-cell proliferation. (A) Knockdown of CYTIP expression was monitored by immunofluorescence staining of mature DCs 24 hours after transfection with unrelated control and CYTIP siRNA, respectively. Original magnification ×63. (B) Expression of surface markers known to be up-regulated on maturation of DCs was analyzed by flow cytometry after transfection of wild-type DCs with control siRNA and specific CYTIP siRNA, respectively. Surface staining of CD80 or CD40 on CD11c+ DCs is depicted in the 2 bottom rows and was found not to be significantly different in CYTIP-silenced versus control DCs. Specific staining for CD11c, CD80, and CD40 is indicated by filled lines; isotype control is illustrated by unfilled lines. The geometric mean of specific staining was quantified and is shown within each fluorescence-activated cell sorter profile. (C) Mature DCs were transfected with control or CYTIP-specific siRNA and cocultivated in presence of the ovalbumin peptide with naive CD4+ OT-II T cells in a 3-D collagen gel. Contact times of individual T-cell/DC pairs were analyzed and were found to be significantly enhanced on silencing of CYTIP expression. (D) Antigen-specific proliferation of CD4+ OT-II T cells on cocultivation with DCs that were left untreated or were transfected with control or CYTIP siRNA was assessed by incorporation of [3H]thymidine. Assays were performed in triplicate. One representative assay of 3 is shown. *P < .05. cpm indicates counts per minute.
Quantification of the interaction time between DCs and naive CD4+ OT-II T cells revealed that T cells stayed in contact with CYTIP-silenced DCs longer than with controls (Figure 6C). In support of our previous findings, prolonged contact duration resulted in significantly reduced T-cell proliferation after cocultivation with antigen-loaded CYTIP-silenced DCs (Figure 6D). Using 2 different approaches, we provide evidence that inactive LFA-1 on DCs might be required for productive DC/T-cell interaction and antigen-specific T-cell activation. The present data suggest that a defined time window of T-cell/APC contact duration exists, with extended contacts inversely correlated with T-cell proliferation and effective immune responses.
Inhibition of active LFA-1 on DCs prevents formation of prolonged contacts with T cells and rescues normal T-cell proliferation
Recent data indicate that LFA-1 on DCs is expressed in an inactive state and has therefore been considered to have no functional importance for DC activity.8,9 Having established that active LFA-1 on DCs prolongs their contact with T cells and thereby impairs T-cell priming, we would speculate that for full DC function, LFA-1 must be expressed in an inactive state. To examine whether inhibition of active LFA-1 rescues DC function, we pretreated antigen-pulsed LFA-1d/d with functional LFA-1 (CD11a/CD18)–blocking antibodies and monitored their interaction with naive CD4+ OT-II T cells in a 3-D collagen matrix. Antibodies directed against CD11a (FD441.8) or CD18 (GAME46) did not significantly influence the mean contact time of wild-type DCs with T cells (Figure 7A), which confirms that in contrast to active LFA-1, wild-type LFA-1 on DCs does not affect DC/T-cell interaction. Inhibition of CD11a or CD18 on DCs that expressed constitutively active LFA-1 reduced their contact time with T cells compared with LFA-1d/d DCs incubated with an antibody control or a nonblocking CD18 antibody (M18/2; Figure 7A). In fact, the mean contact time of LFA-1d/d cells pretreated with an antibody against CD11a (22.6 ± 2.1 minutes) or CD18 (19.6 ± 4.3 minutes) was not significantly different from the contact duration of wild-type DCs with T cells incubated with a control antibody (16.4 ± 1.5 minutes) or a nonblocking CD18 antibody (18.6 ± 4.1 minutes). Thus, inhibition of active LFA-1 on DCs prevents prolonged contacts between LFA-1d/d DCs and T cells. Consistent with previous findings, the blocking of wild-type LFA-1 on DCs did not affect their interaction with naive T cells. Furthermore, pretreatment of LFA-1d/d DCs with inhibitory CD11a or CD18 antibodies restored the T-cell stimulatory capacity of these cells (Figure 7B). These data support the results of the DC phenotyping (Figure 1B; supplemental Figure 1) that indicated that reduced T-cell activation by LFA-1d/d DCs did not result from a less mature DC phenotype or altered DC function.
Inhibition of active LFA-1 on DCs prevents the formation of prolonged contacts and rescues antigen-specific T-cell proliferation. Mature DCs from wild-type and LFA-1d/d mice were left untreated or were pretreated with inhibitory antibodies directed against CD18 (GAME46) or CD11a (FD441.8), with a nonblocking CD18 antibody (M18/2) and an isotype control, respectively. Subsequently, DCs were pulsed with the ovalbumin peptide and cocultivated with naive CD4+ OT-II T cells in a 3-D collagen gel (ratio of 1 DC per 30 T cells). Contact times of individual T cells with wild-type versus LFA-1d/d DCs (A) or DCs transfected with control or CYTIP-specific siRNA (C) were analyzed. Antigen-specific proliferation of CD4+ OT-II T cells was assessed by incorporation of [3H]thymidine (B,D). Assays were performed in triplicate. One representative assay of 3 is shown. ns indicates not significant.
Inhibition of active LFA-1 on DCs prevents the formation of prolonged contacts and rescues antigen-specific T-cell proliferation. Mature DCs from wild-type and LFA-1d/d mice were left untreated or were pretreated with inhibitory antibodies directed against CD18 (GAME46) or CD11a (FD441.8), with a nonblocking CD18 antibody (M18/2) and an isotype control, respectively. Subsequently, DCs were pulsed with the ovalbumin peptide and cocultivated with naive CD4+ OT-II T cells in a 3-D collagen gel (ratio of 1 DC per 30 T cells). Contact times of individual T cells with wild-type versus LFA-1d/d DCs (A) or DCs transfected with control or CYTIP-specific siRNA (C) were analyzed. Antigen-specific proliferation of CD4+ OT-II T cells was assessed by incorporation of [3H]thymidine (B,D). Assays were performed in triplicate. One representative assay of 3 is shown. ns indicates not significant.
Because down-regulation of CYTIP expression in DCs induced prolonged contacts that resulted in reduced T-cell proliferation (Figure 6C,D), and because it has not been shown whether inactivation of CYTIP causes its effect through activation of LFA-1, we examined whether we could rescue antigen-specific T-cell proliferation by blocking LFA-1 on CYTIP-silenced DCs. Pretreatment of DCs with an inhibitory CD18 antibody (GAME46) decreased the contact time between CYTIP-silenced DCs and naive T cells (Figure 7C) and increased T-cell proliferation to a comparable level as DCs transfected with control siRNA (Figure 7D). Confirming these results, we found that CYTIP knockdown had no effect on the stimulatory capacity of CD18-deficient DCs, whereas silencing of CYTIP in wild-type DCs significantly reduced antigen-specific T-cell proliferation (supplemental Figure 4). Taken together, the present data demonstrate that active LFA-1 on DCs is inhibitory for antigen-specific T-cell proliferation and imply that CYTIP expression in mature DCs regulates LFA-1–dependent T-cell release that is a prerequisite for successful T-cell priming.
Discussion
Although the interaction of LFA-1 on T cells with ICAM-1 on APCs is considered to have a prominent role in facilitating T-cell/APC adhesion and antigen-specific T-cell activation,5 very little information is available about the role of LFA-1 on DCs in this context. LFA-1 is believed to be expressed in an inactive state on the DC surface.8,9 However, recent studies suggest that β2-integrins on mature DCs can be activated by physiologic stimuli, such as chemokine-mediated signaling,25 and DCs adhere via LFA-1 to activated endothelium.26 We now provide evidence that controlling the activation status of LFA-1 on DCs may be a key mechanism by which these cells regulate their interaction with T cells. Forcing expression of active LFA-1 on DCs promoted the formation of long-lived DC/T-cell contacts and inversely correlated with T-cell activation, antigen-specific proliferation, and differentiation into Th1 effector cells. We could rescue normal interaction time and T-cell proliferation by inhibition of active LFA-1 on DCs, which indicates that inactive LFA-1 is required for effective T-cell priming and that LFA-1 activity per se does not influence the maturation state or basic function of DCs.
The formation of long-lived contacts between T cells and antigen-bearing DCs is suggested to be mandatory for T-cell activation and clonal expansion.7,22 The high density of TCR27 and the strength of antigenic signals are considered to influence DC/T-cell adhesion, because high-avidity but not low-avidity peptide-MHC complexes on DCs induce prolonged contacts.28 In addition, the antigen dose correlates with the duration of T-cell/APC interaction and T-cell proliferation.29 In the presence of low antigen doses on DCs, T cells are highly motile and engage in serial contacts with different DCs,29 which might facilitate the accumulation of signals.30-32 LFA-1–dependent T-cell binding to ICAM-1 on APCs is known to lower the threshold of T-cell activation in vitro and in vivo.5,33,34 Taken together, the interaction of LFA-1 on T cells with ICAM-1 on DCs appears to regulate the formation of long-lived contacts and thereby influences antigen-specific T-cell activation. Although activation of Mac-1 appeared to have no effect on DC/T-cell interaction,8 we demonstrate here that active LFA-1 on DCs induces T-cell arrest. Expression of active LFA-1 regulated the formation of long-lasting but not short contacts, which suggests that LFA-1 on DCs is dispensable for short-lived antigen-specific T-cell interactions. Similar previous observations demonstrated in vivo that ICAM-1 expression by mature DCs is critical for long-lasting contacts with CD8+ T cells; however, short contacts were not affected by a lack of ICAM-1 on DCs.7 Furthermore, the present data indicate that expression of active LFA-1 on DCs does not induce uncontrolled stickiness to ICAM-1–bearing cells but specifically increases the number of long-lived DC/T-cell contacts.
In contrast to some reports, we demonstrate here that the formation of prolonged contacts induced by active LFA-1 on DCs negatively correlates with antigen-specific T-cell proliferation, which suggests that the formation of sustained T-cell/DC contacts alone might not be sufficient for productive T-cell activation. This hypothesis is supported by the observation that the exact duration of long-lived contacts in vivo appears to be difficult to define and ranges from 6 hours to several days.1,7,22 If it is not the contact duration between APCs and naive T cells that determines successful T-cell activation, LFA-1–mediated signaling within DCs could influence DC cross talk with T cells during antigen presentation. Such an interdependent interaction between DCs and T cells has been observed previously and revealed that CD80/CD86-dependent preactivation of T cells by DCs promotes the formation of stable DC/T-cell contacts and subsequent antigen-specific T-cell proliferation.35 Alternatively, on successful activation of T cells, their release from the DC could be necessary for T-cell proliferation, which implies that T-cell/DC adhesion must be transient. Consistent with this view, integrin-mediated signaling is known to regulate cell division,36-38 and a temporal connection between T-cell detachment from antigen-bearing DCs and T-cell division has been observed in vivo.3 In the present study, expression of active LFA-1 on DCs led to an accumulation of T cells on the DC surface, which indicates that defective LFA-1 deactivation induces a stop signal that prevents T cells from detaching from the APC. Active LFA-1 expressed on T cells previously has been shown to inhibit their de-adhesion from endothelial cells, thus bringing migrating T cells to a halt.17 Recent reports identified cytotoxic T-lymphocyte antigen-4 (CTLA-4), a coreceptor that provides inhibitory signals, as an important negative regulator for T-cell migration.39 Recognition of MHC class II–peptide complexes by T cells locks LFA-1 into its high-affinity conformation,40 which induces a stop signal that stabilizes T-cell/APC interactions. In addition, termination of TCR signaling is known to promote T-cell detachment from the APC.22 T-cell de-adhesion depends on cytoskeletal reorganization, and nonmuscle myosin heavy chain IIA has been shown to provide the contractile force needed to disrupt LFA-1/ICAM-1 binding, which allows detachment of the trailing edge of migrating T cells.41 Recently, inhibition of myosin IIA in T cells has been demonstrated to interfere with immunologic synapse stability.42 Considered together, the present data and results from the literature emphasize a crucial role for LFA-1/ICAM-1–mediated T-cell de-adhesion from the DC and suggest that the dynamic regulation of LFA-1 activity on DCs controls their contact with T cells and subsequently influences antigen-specific T-cell proliferation. In addition, other surface receptors might be involved in the formation of productive T-cell/DC interactions. For example, expression of neuropilin-1 in T cells has been demonstrated to induce long-lived interactions with immature DCs and increased the sensitivity of T cells to their cognate antigen.43
The formation of long-lasting contacts between antigen-bearing DCs and naive T cells promotes IFN-γ and IL-2 production by activated T cells in vivo.1,32 In addition, IFN-γ release by effector CD8+ T cells has been described to depend on ICAM-1–dependent stable T-cell/DC contacts in vivo.7 We demonstrate here that although it induces long-lived T-cell/DC interactions, active LFA-1 on DCs negatively affects intracellular levels of IL-2 and IFN-γ, which are known to be produced by activated Th1 effector cells. In addition, locking LFA-1 into an active state specifically reduced mRNA expression of Th1-driven genes. Because Th1 effector function is considered to facilitate delayed-type hypersensitivity responses,44 we found reduced delayed-type hypersensitivity reactions on transfer of LFA-1d/d DCs. The present results indicate that not only prolonged contact duration but also defective LFA-1 deactivation might be responsible for reduced T-cell activation by LFA-1d/d DCs. In contrast, generation of Th2 and regulatory T cells appeared to be unaffected by active LFA-1 on DCs in vitro. Thus, expression of active LFA-1 per se does not induce regulatory T cells, which confirms previous studies that revealed that expression of active β2-integrins on DCs does not affect proliferation of CD4+CD25+ T cells.8 The present data further illustrate that induction of prolonged contacts between APCs and T cells is probably not the only factor that controls the generation of regulatory T cells, as has been implied for the interaction of T cells with naive B cells.45
Recent publications suggested that T-cell/DC engagement triggers clustering of inactive LFA-1 on the DC membrane.46 It remained unclear, however, how deactivation of LFA-1 in DCs is accomplished. In the present study, knockdown of CYTIP in mature DCs increased the duration of long-lived contacts with T cells and reduced T-cell proliferation, and this was found to depend on LFA-1. CYTIP has been shown to colocalize with cytohesin-1 in the cytoplasm of DCs (supplemental Figure 3),15 and levels of CYTIP mRNA and protein are up-regulated on maturation of DCs (supplemental Figure 5A-C).15 These data therefore indicate that CYTIP functions as a regulator of LFA-1 activity in DCs, controlling DC/T-cell de-adhesion, and that it allows productive antigen-specific T-cell proliferation to occur.
Previously, Hofer et al24 found reduced priming of CD8+ T cells in 4 patients when only a fraction of CYTIP-silenced DCs were pulsed with antigen; however, in a setting in which all DCs were antigen loaded, they detected a slightly enhanced T-cell proliferation induced by CYTIP-silenced DCs, which suggests that in the situation of a productive DC/T-cell interaction, a more stable contact with CYTIP-silenced DCs positively regulates T-cell proliferation. In contrast, the present results reveal that even in the presence of a high antigen dose, increased DC/T-cell adhesion reduces the strength of T-cell activation. However, these apparent discrepancies are likely to result from substantial differences in the experimental setup between the 2 studies. Whereas data from Hofer et al24 were obtained under conditions of static DC adhesion to T cells in a 2-dimensional environment, the experiments of the present study were performed in a 3-D environment that allowed assessment of active cell-cell contacts and was designed to mimic DC/T-cell interaction in the lymph node parenchyma in vivo.10,21 In addition, it has been shown that although leukocyte migration in a 2-dimensional environment is dominated by integrin-mediated events, migration in 3-D appeared to be integrin independent.47 It is tempting to speculate that in a 2-D experimental setting, induction of active LFA-1 by CYTIP knockdown not only primarily affects T-cell proliferation but might also have an impact on DC crawling on T cells. In a similar approach, mysosin9b-deficient T cells showed a reduced proliferative capacity in a 3-D setting; however, in 2 dimensions, their proliferation was enhanced (S.H., unpublished observation, April 2009).
Taken together, in vitro and in vivo results of the present study indicate that regulation of LFA-1 activity on DCs is a crucial event during DC/T-cell conjugate formation and suggest that mature DCs express high levels of CYTIP to ensure the presence of inactive LFA-1 required for T-cell release and subsequent T-cell priming. Maturation of DCs has been demonstrated to be associated with a reduced ability to adhere to recombinant ICAM-148 and fibronectin.24 In contrast, immature DCs and naive B cells, both of which are believed to be inefficient in antigen-specific T-cell activation, express only low amounts of CYTIP.49 Still, naive B cells form long-lasting contacts with T cells10 that have been described to specifically induce differentiation into regulatory T cells.45 An additional in vivo mechanism to regulate LFA-1 activity on DCs must exist, because ovalbumin-loaded CYTIP-deficient DCs have been demonstrated to induce CD4+ T-cell proliferation to a similar extent as wild-type DCs.50
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Karin Scharffetter-Kochanek (University of Ulm, Germany) for providing CD18−/− mice and Thomas Blankenstein (Max-Delbrück Center Berlin, Germany) for providing the OT-I and OT-II mice. We thank Ingrid Tubbe and Nicole Voltz (University of Mainz, Germany) for technical assistance; Mathias Krummen (University of Mainz) for discussion and research design; Cora Schild (University of Bonn, Germany) for experimental support; Karin Loser (University of Münster, Germany), Martin Wild (MPI for Molecular Biomedicine, Münster, Germany), and Georg Varga (University of Münster, Germany) for technical advice; and Dirk Busch (TU München, Germany) for his comments and suggestions on the manuscript.
This work was supported by German Research Council grants Gr1022/9-3, SFB629/B3, and Gr1022/10-1 (S.G.).
Authorship
Contribution: S.B. performed experiments, analyzed the data, and supported M.L. and S.G. in research design; S.H. and P.S. performed experiments and analyzed the data; W.K. and B.H. contributed with research reagents; S.G. designed experiments and acquired funding for the project; and M.L. designed the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Melanie Laschinger, Technische Universität München, Department of Surgery, Ismaninger Straße 22, 81675 Munich, Germany; e-mail: laschinger@chir.med.tu-muenchen.de.
References
Author notes
S.B. and S.H. contributed equally to this study.


![Figure 3. Active LFA-1 on DCs negatively influences T-cell proliferation. Mature DCs from wild-type (WT) and LFA-1d/d mice were loaded with ovalbumin peptides and cocultivated in different concentrations with naive CD4+ OT-II (A) and CD8+ OT-I (B) in a 3-D collagen gel for 3 days. Cell proliferation was assessed by incorporation of [3H]thymidine. Assays were performed in triplicate. One representative assay of 3 is shown. cpm indicates counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/11/10.1182_blood-2009-05-224428/5/m_zh89991056770003.jpeg?Expires=1765936145&Signature=ezchrdTtv4dBO~Xjp2Qg58HKtWN~U---44sfwCZwxwCCImmetUJ2stCqPXh0bQOmyYGMBXOJ1oVfK~AUNvzM5Lz~sUQaaW~gt8zKbIm8edjCSdAWP9A3xlMppEiFGKhj3ETo22Ys9U3-ncF~tJ2lZIwmy5aEe9yZ-tyVMqzjpa49gFGHepG9S26FeyQ6g1BfMsd4SbgLVINTU5o6CtWlhaIu-Yfoje3W6yyvvt3jFO5PQjzsccKY4dWs4NUhJm4ot86E3WDsOmAvf9bPHetQoR2xoGf-Xog6B684tueNx~VogpCPUr0TxKydHJ~JUk3B8Xpg0ows773ajPn5PrqVSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Active LFA-1 on DCs interferes with T-cell activation and generation of Th1 cells. (A) OT-I T cells were cocultivated for 4 hours with wild-type (WT) or LFA-1d/d DCs loaded with different doses of the ovalbumin peptide SIINFEKL in a 3-D collagen matrix. On isolation from the matrix, expression of the Vα2-chain of the TCR was measured by flow cytometry. Mean fluorescence intensity (MFI) of Vα2 on CD8+ T cells is depicted (n = 3). Amount of TCR down-regulation by wild-type or LFA-1d/d DCs was found to be insignificant. (B) OT-I T cells were activated by wild-type or LFA-1d/d DCs pulsed with different doses of SIINFEKL for 3 days in parallel to experiments performed in panel A. Proliferation was measured by incorporation of [3H]thymidine (n = 2). (C) OT-II T cells and ovalbumin peptide–loaded DCs were isolated from the collagen matrix after 24 hours of coculture. Total RNA was extracted, and quantitative real-time PCR was performed. RNA expression levels were normalized to expression of endogenous β-actin mRNA. Relative expression of IL-2 mRNA of wild-type DCs cocultivated with OT-II T cells in the absence of peptide was equalized to 1 (n = 3). *P < .05. (D) Naive CD4+ OT-II cells were cocultivated for 24 hours with DCs in the presence or absence of ovalbumin peptide in a 3-D collagen matrix. Expression of intracellular IFN-γ was analyzed by flow cytometry. The number of IFN-γ-positive CD4+ T cells was quantified (n = 2). *P < .05. (E) Total RNA from CD4+ OT-II T cells cocultivated with ovalbumin-loaded DCs in a 3-D collagen gel for 24 hours was extracted, and quantitative real-time PCR was performed. RNA expression levels were normalized to expression of endogenous β-actin mRNA. Relative expression of Tbet (n = 4), GATA3 (n = 4), and FoxP3 (n = 3) mRNA of wild-type DCs cocultivated with OT-II T cells was equalized to 1. *P < .05. cpm indicates counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/11/10.1182_blood-2009-05-224428/5/m_zh89991056770004.jpeg?Expires=1765936145&Signature=bzJXhxyriSj1USz7P3LZ2n7GMEE8-SAGzg6JkMsAOsLioT7RXo5MYRWxHBWjyEEsdiAlF8DMbB0LH5LZc0zxfFoX1I-g4InhM34i6L3ZgYI4aZSu4jNr67UcaQj8SckeTpiwb8OCeKhL0svhV7Cl1q0bv0oCOOLELKGjCgkgoD4A73E6u48M5i8Igz8Ih~hgQmn5wU43IL9ydp35L0F3tQ2uD-~Jtg0QdXtQAWiKj5wdyuznH8SlP0KiOer50TP5tNtkKTmitODr8sIkuHPH8jzcwvdQk-gBj3ijUzU8yW8nZ~DrfG1LytcclxszJSRG4V9mbSH~trsV17D~jiQf5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. CYTIP silencing in DCs induces prolonged contacts with naive T cells and impairs antigen-specific T-cell proliferation. (A) Knockdown of CYTIP expression was monitored by immunofluorescence staining of mature DCs 24 hours after transfection with unrelated control and CYTIP siRNA, respectively. Original magnification ×63. (B) Expression of surface markers known to be up-regulated on maturation of DCs was analyzed by flow cytometry after transfection of wild-type DCs with control siRNA and specific CYTIP siRNA, respectively. Surface staining of CD80 or CD40 on CD11c+ DCs is depicted in the 2 bottom rows and was found not to be significantly different in CYTIP-silenced versus control DCs. Specific staining for CD11c, CD80, and CD40 is indicated by filled lines; isotype control is illustrated by unfilled lines. The geometric mean of specific staining was quantified and is shown within each fluorescence-activated cell sorter profile. (C) Mature DCs were transfected with control or CYTIP-specific siRNA and cocultivated in presence of the ovalbumin peptide with naive CD4+ OT-II T cells in a 3-D collagen gel. Contact times of individual T-cell/DC pairs were analyzed and were found to be significantly enhanced on silencing of CYTIP expression. (D) Antigen-specific proliferation of CD4+ OT-II T cells on cocultivation with DCs that were left untreated or were transfected with control or CYTIP siRNA was assessed by incorporation of [3H]thymidine. Assays were performed in triplicate. One representative assay of 3 is shown. *P < .05. cpm indicates counts per minute.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/11/10.1182_blood-2009-05-224428/5/m_zh89991056770006.jpeg?Expires=1765936145&Signature=0mbrn9pUr8igL8-AdJp9RgI62tf5976vVOZ5C0SYWwOK~9eDH-1kZFMBLuU2yfiNF1jnmQXZ--8PKo4QF109epYx1QEPQGX92xAYL3nxpgDzM7~9WRqLcvLmRIvA8Z3xFjyTxvhrGM1k0CuKsK3GCGgLZ8GRFH1-firxYHy0KlsfakCM7-ptHMILgDGonyxOHOK0nGKvI~PMjypBdYpMvX1CLnMdv0qS2Sgo3wSJOQTP3J7qLXEpD1RRqGEZxt-G7LBC69aGRh4JQj81eKq-33k9W-crl~FGh2NvsH~hmhXtL-xV6fTQ6yR2ubpqPFGxH2FubCdRLkPfGepFsU3SxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Inhibition of active LFA-1 on DCs prevents the formation of prolonged contacts and rescues antigen-specific T-cell proliferation. Mature DCs from wild-type and LFA-1d/d mice were left untreated or were pretreated with inhibitory antibodies directed against CD18 (GAME46) or CD11a (FD441.8), with a nonblocking CD18 antibody (M18/2) and an isotype control, respectively. Subsequently, DCs were pulsed with the ovalbumin peptide and cocultivated with naive CD4+ OT-II T cells in a 3-D collagen gel (ratio of 1 DC per 30 T cells). Contact times of individual T cells with wild-type versus LFA-1d/d DCs (A) or DCs transfected with control or CYTIP-specific siRNA (C) were analyzed. Antigen-specific proliferation of CD4+ OT-II T cells was assessed by incorporation of [3H]thymidine (B,D). Assays were performed in triplicate. One representative assay of 3 is shown. ns indicates not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/11/10.1182_blood-2009-05-224428/5/m_zh89991056770007.jpeg?Expires=1765936145&Signature=yhrxa~dgPEKGYzu1jCkpVNjvmpR6ypbJc2Pr3iVeG4TuFA4Zm7Kz1i5dZ3~37RPj8VDnkA37IA4HDXyt5cVo74xoCXtx0wZysYbHROVJ1gvfc-jseh4JECj3N6Pl-JVteD8nPC7nIOuuyGPCWbm8VeHk9cy8Ar-fEIR5VjaTcxwprvZvco~QpCjvAzPWPr6g0cdzgA8pevFQr2zDbQZlEC034DyHiGndeaAcESmHg7AtABtmQf8UrODcmdPm1QgQrqjpf4aeNky1vu~s3IoThQ6KsNbt5tQ-r~r5ExdnxKjr8fnOJ6KhXm-lf89FYp~CRPcWUzeSzupRKUsiTdbfSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal