Abstract
We analyzed risk factors influencing outcomes after related (R) human leukocyte antigen-identical cord blood transplantation (CBT) for 147 patients with malignancies reported to Eurocord–European Group for Blood and Marrow Transplantation. CBT has been performed since 1990; median follow-up was 6.7 years. Median patient age was 5 years. Acute leukemia was the most frequent diagnosis (74%). At CBT, 40 patients had early, 70 intermediate, and 37 advanced disease. CB grafts contained a median of 4.1 × 107/kg total nucleated cells (TNCs) after thawing. The cumulative incidence (CI) of neutrophil recovery was 90% at day +60. CIs of acute and chronic graft-versus-host disease (GVHD) were 12% and 10% at 2 years, respectively. At 5 years, CIs of nonrelapse mortality and relapse were 9% and 47%, respectively; the probability of disease-free survival (DFS) and overall survival were 44% and 55%, respectively. Among other factors, higher TNCs infused was associated with rapid neutrophil recovery and improved DFS. The use of methotrexate as GVHD prophylaxis decreased the CI of engraftment. Patients without advanced disease had improved DFS. These results support banking and use of CB units for RCBT. Cell dose, GVHD prophylaxis not including methotrexate, and disease status are important factors for outcomes after RCBT.
Introduction
Since the publication of the first related (R) cord blood transplantation (CBT) in a child with Fanconi anemia,1 umbilical cord blood (CB) has been well recognized and widely used as a source of hematopoietic stem cells for the treatment of both nonmalignant and malignant disorders in pediatric and adult patients. In children, despite a decreased incidence of neutrophil recovery after related human leukocyte antigen (HLA)–identical CBT compared with bone marrow transplantation (BMT), survival according to disease type after CBT (malignant or nonmalignant diseases) has been shown to be comparable with that after BMT.2 Moreover, incidences of acute and chronic graft-versus-host disease (GVHD) were decreased after CBT compared with BMT.2 However, follow-up was short (2 years) in this analysis, and long-term outcomes were not described.2 RCBT in hemoglobinopathies, namely sickle cell disease and thalassemia, result in relatively low toxicity, particularly when methotrexate (MTX) is not used for GVHD prophylaxis.3
Few studies have reported outcomes after RCBT for patients with malignant disorders.2,4-6 A Eurocord study reported risk factors for outcomes in 102 children with acute leukemia (AL) who received a CB transplant from either a related donor (n = 42; 12 with HLA-mismatched donor and 30 with HLA-identical donor) or unrelated donor (n = 60); in that study, better disease-free survival (DFS) was reported in patients who received a transplant in first or second complete remission.5 In a subsequent preliminary analysis, we showed that outcomes after HLA-identical RCBT were better than after HLA-mismatched RCBT in patients with malignancies.7 In the present study, we analyzed risk factors for outcomes solely after related HLA-identical CBT in malignancies and describe long-term outcomes, particularly relapse. There were 147 patients who underwent RCBT and reported to Eurocord–European Group for Blood and Marrow Transplantation (EBMT) with complete data over the past 2 decades, of whom 45 patients were included in previous studies.2,4,5
Methods
Data collection
Data on patient, disease, CB unit characteristics, and outcomes of RCBT performed from October 26, 1990, to September 22, 2008, were collected with the use of the standardized Eurocord CBT questionnaire (100-day report and yearly follow-up forms). Data were entered in the Eurocord database after validation by clinical research physicians. For the purposes of this study, each transplantation center was contacted individually for participation in this study. They were asked to complete missing data and to update patient follow-up. Approval was given by all participating transplantation centers for this study. According to EBMT rules, patients gave informed consent for data entry into the EBMT and Eurocord registry databases in accordance with the Declaration of Helsinki.
Patient selection and follow-up
Among CBTs performed until December 31, 2008, and reported to the Eurocord or EBMT registries, we selected patients with a diagnosis of malignant disease who had a first allogeneic single HLA-identical (HLA-A, -B and -DRB1 antigenic typing) unmanipulated CBT from a related donor. One hundred sixty-two cases fulfilled inclusion criteria, and 15 cases were excluded due to lack of response from the transplantation centers to participate in this study. We thus included 147 cases with sufficient patient, disease, CB characteristics, and posttransplantation outcomes (minimum follow-up of 100 days after CBT) in this analysis. CBT was performed over a period of 18 years, from 1990 to 2008 (median year of transplantation being 2000), in 67 different transplantation centers (each reporting between 1 and 13 cases) and 25 different countries worldwide (excluding the United States and Japan), including 112 CBTs performed in European countries. Median follow-up was 6.7 years, ranging from 7 months to 18 years (only 3 patients having a follow-up < 1 year). Of 83 live patients, follow-up was updated to 2009 for 59 patients, to 2008 for 10 patients, and to previous years for 14 patients.
Definitions
Disease status was classified according to criteria from the Center for International Blood and Marrow Transplant Research.8 Early disease included cases of AL in first complete remission (CR), lymphomas in first CR, and chronic myeloid leukemia (CML) in first chronic phase. Intermediate disease included cases of AL in at least second CR, lymphomas in at least second CR, or CML in accelerated phase. Advanced disease included AL not in CR (refractory), lymphomas not in CR, CML in blastic crisis or at least second chronic phase, all myelodysplastic disorders, and solid tumors.
A myeloablative conditioning was defined as a regimen containing either total body irradiation (TBI) with a dose of more than 6 Gy, a dose of oral busulfan of more than 8 mg/kg, a dose of intravenous busulfan of more than 6.4 mg/kg, or a dose of treosulfan of at least 12 g/m2. Any other conditioning regimen was considered of reduced intensity.
Neutrophil recovery was defined as the first of 3 consecutive days when the absolute neutrophil count was 0.5 × 109/L or higher, irrespective of donor hematopoiesis. Platelet recovery was defined as the first of 7 consecutive days when platelet count reached 20 × 109/L or higher, independent of platelet transfusions and irrespective of the degree of donor hematopoiesis. Full-donor chimerism was defined as at least 95% leucocytes of donor origin in peripheral blood or marrow samples, measured by different techniques according to transplantation centers. Autologous reconstitution was defined as at least 95% leucocytes of recipient origin, and there was mixed chimerism when greater than 5% but less than 95% leucocytes were of donor origin. Acute GVHD was diagnosed and graded according to standard criteria.9 Chronic GVHD was also diagnosed according to standard criteria.10 Only patients with sustained donor engraftment surviving more than 100 days after CBT were considered at risk of developing chronic GVHD. Nonrelapse mortality (NRM) was defined as any death not related to relapse. Relapse was defined on the basis of morphologic recurrence of original disease. Response to donor lymphocyte infusion (DLI) or second transplantation was defined as no relapse or death occurring after treatment until the date of last follow-up. Overall survival (OS) was calculated from transplantation to death from any cause, and DFS was defined as the time from transplantation to either relapse or death, whichever occurred first.
Statistical analysis
The duration of follow-up was the time to last assessment for survivors. For each continuous variable, the study population was initially split into quartiles and in 2 groups by the median. The median value was found to be the best cutoff for analysis of all outcomes with one exception: for analysis of OS, the years of transplantation were best split into 3 categories (< 1997, 1997-2003, and ≥ 2004). Variables considered in risk factor analysis for neutrophil recovery, relapse, DFS, and OS were year of transplantation (with < 1997 vs ≥ 1997 and < 2004 vs ≥ 2004 for OS and the median year of transplantation of 2000 as a cutoff for all other outcomes), recipient age (with the median age of 5 years as a cutoff), sex (female vs male), cytomegalovirus serologic status (negative vs positive), previous autologous transplantation (no vs yes), disease status category according to International Bone Marrow Transplant Registry criteria (early, intermediate, advanced), ABO match (match, minor incompatibility, major incompatibility), sex match (match vs mismatched), infused cell dose in total nucleated cells (TNCs) per kilogram (with the median infused TNCs of 4.1 × 107/kg as a cutoff), use of TBI vs busulfan in myeloablative conditioning, use of MTX for GVHD prophylaxis (no vs yes). A subgroup risk factor analysis for patients with AL was performed with the use of the same methods, variables, and statistics except that the remission status (CR1, CR2, CR3, and refractory disease, as well as state of CR vs no remission) was used instead of the Center for International Blood and Marrow Transplant Research disease status categories. An association between categorical variables was tested by the chi-square test. The confidence intervals (CIs) of neutrophil recovery, platelet recovery, and chronic GVHD were calculated with the use of death from any cause and second transplantations as competing risks, and the CIs of acute GVHD was calculated only with the use of death as a competing risk. Second transplantations, as well as death in remission and relapse, were competing risks for calculating CI of relapse and NRM, respectively. Univariate analysis for these outcomes was done with the use of the Fine and Gray test. Probabilities of DFS and OS were calculated with the use of the Kaplan-Meier estimate, and the log-rank test was used for univariate comparisons. Multivariate analyses were performed with Cox proportional hazards regression model for DFS and OS, and Fine and Gray proportional hazards regression model was used for subdistribution of other outcomes.11 Variables that reached a P value of .20 after univariate analysis were included in the initial models, and variables were eliminated one at a time in a stepwise fashion to only keep variables that reached a P value of .05 or less into the final models. All P values were 2-sided, with a type I error rate fixed at 0.05. Statistical analyses were performed with SPSS Version 18 (SPSS Inc) and S-Plus Version 6.1 (MathSoft) software.
Results
Patient, disease, graft, and transplantation characteristics
Patient and disease characteristics are described in Table 1. All patients were children except 3, aged 18, 19, and 31 years, respectively. The majority (74%) of patients had AL. Intermediate disease patients constituted the largest risk group. For the 109 patients with AL, there were 4 cases of secondary AL. Among the 82 patients with acute lymphoblastic leukemia (ALL), 54 had available cytogenetics; 7 patients had Philadelphia chromosome ALL. Among the 20 patients with acute myeloid leukemia, 12 had available cytogenetics, including 6 with high-risk anomalies (namely complex karyotype or monosomy 7).
Patient and disease characteristics
| Characteristic . | Value . | n . |
|---|---|---|
| Male sex, n (%) | 90 (61) | 147 |
| Median age, y (range) | 5.0 (1.0-31.6) | 147 |
| Median weight, kg (range) | 18.4 (8-69) | 146 |
| Previous autologous transplantation, n (%) | 11 (7.6) | 145 |
| Positive CMV serology, n (%) | 65 (46) | 141 |
| Diagnosis | 147 | |
| Acute leukemia | 109 (74) | |
| ALL, n | 82 | |
| AML, n | 20 | |
| Biphenotypic, n | 6 | |
| Undifferentiated, n | 1 | |
| Myelodysplastic syndrome, n (%) | 17 (12) | |
| JMML, n | 10 | |
| Other MDS, n | 3 | |
| Unspecified MDS, n | 4 | |
| Chronic myeloid leukemia, n (%) | 12 (8) | |
| Lymphoproliferative disorder, n (%) | 3 (2) | |
| Anaplastic large cell lymphoma, n | 1 | |
| Burkitt lymphoma, n | 2 | |
| Solid tumor, n (%) | 6 (4) | |
| Neuroblastoma, n | 5 | |
| Ewing sarcoma, n | 1 | |
| Disease status | 147 | |
| Early, n (%) | 40 (27) | |
| Intermediate, n (%) | 70 (48) | |
| Advanced, n (%) | 37 (25) | |
| Median time from diagnosis to RCBT, mo (range) | 17.0 (0.8-117.8) | 147 |
| Early | 8.9 (0.8-82.5) | |
| Intermediate | 36.0 (5.7-117.8) | |
| Advanced | 12.2 (1.4-52.2) | |
| Remission status in patients with AL | 109 | |
| First CR, n (%) | 31 (28) | |
| Second CR, n (%) | 51 (47) | |
| Third or subsequent CR, n (%) | 13 (12) | |
| Refractory disease, n (%) | 14 (13) | |
| Median time from diagnosis to RCBT in patients with AL patients, mo (range) | 22.2 (0.8-117.8) | 109 |
| First CR | 7.8 (0.8-23.2) | |
| Second CR | 33.8 (5.7-88.5) | |
| Third or subsequent CR | 48.5 (16.1-117.8) | |
| Refractory disease | 15.0 (6.0-45.2) | |
| Median time from last CR to RCBT in patients with AL, mo (range) | 3.8 (0.2-25.6) | 82 |
| First CR | 6.8 (0.7-15.5) | |
| Second CR | 3.2 (0.4-25.6) | |
| Third or subsequent CR | 2.2 (0.2-12.0) | |
| Refractory disease | NA | |
| Median length of first CR in patients with AL beyond first CR, mo (range) | 26.3 (2.2-80.4) | 59 |
| Characteristic . | Value . | n . |
|---|---|---|
| Male sex, n (%) | 90 (61) | 147 |
| Median age, y (range) | 5.0 (1.0-31.6) | 147 |
| Median weight, kg (range) | 18.4 (8-69) | 146 |
| Previous autologous transplantation, n (%) | 11 (7.6) | 145 |
| Positive CMV serology, n (%) | 65 (46) | 141 |
| Diagnosis | 147 | |
| Acute leukemia | 109 (74) | |
| ALL, n | 82 | |
| AML, n | 20 | |
| Biphenotypic, n | 6 | |
| Undifferentiated, n | 1 | |
| Myelodysplastic syndrome, n (%) | 17 (12) | |
| JMML, n | 10 | |
| Other MDS, n | 3 | |
| Unspecified MDS, n | 4 | |
| Chronic myeloid leukemia, n (%) | 12 (8) | |
| Lymphoproliferative disorder, n (%) | 3 (2) | |
| Anaplastic large cell lymphoma, n | 1 | |
| Burkitt lymphoma, n | 2 | |
| Solid tumor, n (%) | 6 (4) | |
| Neuroblastoma, n | 5 | |
| Ewing sarcoma, n | 1 | |
| Disease status | 147 | |
| Early, n (%) | 40 (27) | |
| Intermediate, n (%) | 70 (48) | |
| Advanced, n (%) | 37 (25) | |
| Median time from diagnosis to RCBT, mo (range) | 17.0 (0.8-117.8) | 147 |
| Early | 8.9 (0.8-82.5) | |
| Intermediate | 36.0 (5.7-117.8) | |
| Advanced | 12.2 (1.4-52.2) | |
| Remission status in patients with AL | 109 | |
| First CR, n (%) | 31 (28) | |
| Second CR, n (%) | 51 (47) | |
| Third or subsequent CR, n (%) | 13 (12) | |
| Refractory disease, n (%) | 14 (13) | |
| Median time from diagnosis to RCBT in patients with AL patients, mo (range) | 22.2 (0.8-117.8) | 109 |
| First CR | 7.8 (0.8-23.2) | |
| Second CR | 33.8 (5.7-88.5) | |
| Third or subsequent CR | 48.5 (16.1-117.8) | |
| Refractory disease | 15.0 (6.0-45.2) | |
| Median time from last CR to RCBT in patients with AL, mo (range) | 3.8 (0.2-25.6) | 82 |
| First CR | 6.8 (0.7-15.5) | |
| Second CR | 3.2 (0.4-25.6) | |
| Third or subsequent CR | 2.2 (0.2-12.0) | |
| Refractory disease | NA | |
| Median length of first CR in patients with AL beyond first CR, mo (range) | 26.3 (2.2-80.4) | 59 |
CMV indicates cytomegalovirus; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; JMML, juvenile myelomonocytic leukemia; MDS, myelodysplastic syndrome; RCBT, related cord blood transplantation; AL, acute leukemia; and CR, complete remission.
Graft and transplantation characteristics are detailed in Table 2. All CB donors were siblings except one who was a patient's child. The CB unit was transplanted within a median of 3.6 months after birth of the CB donor. The patient's diagnosis antedated the birth of the CB donor by a median of 12 months (0-101 months). There were 60 cases in which this difference was 9 months or less, indicating that the patient's mother was pregnant with the CB donor at the time of the patient's diagnosis. In 82 cases, this difference was more than 9 months, meaning that the CB donor's conception took place after the patient's initial diagnosis. All CB units, except for one that was infused as a fresh product, were cryopreserved at the local hospital cell therapy laboratory or at the nearest public cord blood bank.
Donor and transplantation characteristics
| Characteristic . | Value . | n . |
|---|---|---|
| Median age difference between patient and CB donor, y (range) | 4.1 (0.9-30.7) | 142 |
| Median storage time of the CB unit, mo (range) | 3.6 (0-66.5) | 142 |
| Early disease | 3.0 (1.0-53.6) | |
| Intermediate disease | 5.7 (0-66.5) | |
| Advanced disease | 2.8 (0.8-10.4) | |
| Median total nucleated cells at collection, ×108 (range) | 10.8 (1.3-36.3) | 127 |
| Median total nucleated cells/kg at collection, ×107/kg (range) | 5.1 (1.0-34.6) | 126 |
| Median total nucleated cells/kg at infusion, ×107/kg (range) | 4.1 (0.7-34.6) | 141 |
| ABO compatibility | 143 | |
| Compatible, n (%) | 97 (68%) | |
| Minor incompatibility, n (%) | 16 (11%) | |
| Major incompatibility, n (%) | 30 (21%) | |
| Sex matching (CB/patient) | 146 | |
| Male/male or female/female, n (%) | 80 (55%) | |
| Male/female, n (%) | 34 (23%) | |
| Female/male, n (%) | 32 (22%) | |
| Conditioning regimen | 147 | |
| Reduced intensity, n (%) | 4 (3%) | |
| Myeloablative, n (%) | 143 (97%) | |
| With TBI | 72 | |
| With busulfan (or treosulfan)* | 71 | |
| Antithymocyte globulin, n (%) | 11 (8%) | |
| Use of growth factors after RCBT | 125 | |
| None, n (%) | 39 (31%) | |
| G-CSF, n (%) | 78 (62%) | |
| GM-CSF, n (%) | 5 (4%) | |
| G-CSF and GM-CSF, n (%) | 1 (1%) | |
| G-CSF and EPO, n (%) | 2 (2%) | |
| Median day of onset of growth factors after RCBT (range) | Day (day 0-37) | 79 |
| GVHD prophylaxis | 147 | |
| None, n (%) | 3 (2%) | |
| Steroids alone, n (%) | 2 (1%) | |
| Calcineurin inhibitor-based , n (%) | 142 (97%) | |
| CsA alone, n | 104 | |
| CsA + MMF, n | 3 | |
| CsA + MTX, n | 20 | |
| CsA + MTX + steroids, n | 1 | |
| CsA + steroids, n | 13 | |
| Tacrolimus + steroids, n | 1 |
| Characteristic . | Value . | n . |
|---|---|---|
| Median age difference between patient and CB donor, y (range) | 4.1 (0.9-30.7) | 142 |
| Median storage time of the CB unit, mo (range) | 3.6 (0-66.5) | 142 |
| Early disease | 3.0 (1.0-53.6) | |
| Intermediate disease | 5.7 (0-66.5) | |
| Advanced disease | 2.8 (0.8-10.4) | |
| Median total nucleated cells at collection, ×108 (range) | 10.8 (1.3-36.3) | 127 |
| Median total nucleated cells/kg at collection, ×107/kg (range) | 5.1 (1.0-34.6) | 126 |
| Median total nucleated cells/kg at infusion, ×107/kg (range) | 4.1 (0.7-34.6) | 141 |
| ABO compatibility | 143 | |
| Compatible, n (%) | 97 (68%) | |
| Minor incompatibility, n (%) | 16 (11%) | |
| Major incompatibility, n (%) | 30 (21%) | |
| Sex matching (CB/patient) | 146 | |
| Male/male or female/female, n (%) | 80 (55%) | |
| Male/female, n (%) | 34 (23%) | |
| Female/male, n (%) | 32 (22%) | |
| Conditioning regimen | 147 | |
| Reduced intensity, n (%) | 4 (3%) | |
| Myeloablative, n (%) | 143 (97%) | |
| With TBI | 72 | |
| With busulfan (or treosulfan)* | 71 | |
| Antithymocyte globulin, n (%) | 11 (8%) | |
| Use of growth factors after RCBT | 125 | |
| None, n (%) | 39 (31%) | |
| G-CSF, n (%) | 78 (62%) | |
| GM-CSF, n (%) | 5 (4%) | |
| G-CSF and GM-CSF, n (%) | 1 (1%) | |
| G-CSF and EPO, n (%) | 2 (2%) | |
| Median day of onset of growth factors after RCBT (range) | Day (day 0-37) | 79 |
| GVHD prophylaxis | 147 | |
| None, n (%) | 3 (2%) | |
| Steroids alone, n (%) | 2 (1%) | |
| Calcineurin inhibitor-based , n (%) | 142 (97%) | |
| CsA alone, n | 104 | |
| CsA + MMF, n | 3 | |
| CsA + MTX, n | 20 | |
| CsA + MTX + steroids, n | 1 | |
| CsA + steroids, n | 13 | |
| Tacrolimus + steroids, n | 1 |
CB indicates cord blood; TBI, total body irradiation; CSF, colony-stimulating factor; G, granulocyte; GM, granulocyte macrophage; EPO, erythropoietin; CsA, cyclosporine A; MMF, mycophenolate mofetil; MTX, methotrexate; and RCBT, related cord blood transplantation.
Only 1 patient received treosulfan at a dose of 12 g/m2.
The number of CD34+ cells collected or infused was measured in only a minority of cases (data not shown). Nearly all patients (97%) received a myeloablative conditioning regimen with half based on TBI and half based on busulfan (one patient received treosulfan at a dose of 12 g/m2). Eleven patients (8%) received antithymocyte globulin. GVHD prophylaxis was based on a calcineurin inhibitor (cyclosporine or tacrolimus) for nearly all patients; 15% of patients received MTX in addition to cyclosporine.
Hematopoietic reconstitution
One hundred thirty-three patients reached a neutrophil count of at least 0.5 × 109/L, within a median time of 24 days (range, 4-73 days). The CI of neutrophil recovery at day +60 was 90% plus or minus 3%. Of those 133 patients, chimerism studies were performed and reported in 108 patients: 93 patients (86%) had full donor chimerism, 11 patients (10%) had mixed chimerism, and 4 patients (4%) had autologous reconstitution. Of the 18 patients who did not achieve neutrophil recovery (n = 14) or who had autologous reconstitution (n = 4), only 2 were alive at last follow-up: one at 13 years of follow-up, after receiving an autologous stem cell transplant on day +43, and the other at 2.5 years of follow-up after receiving chemotherapy. The other 16 patients died early after transplantation, at a median of 30 days (range, 5-514 days), including one patient who died on day +119 after receiving a second transplant with bone marrow from the same donor on day +31. Of the 12 patients with mixed chimerism, 8 are alive, including 1 patient at 2.2 years of follow-up after receiving a haplo-identical stem cell transplant from his father on day +72 for secondary graft failure.
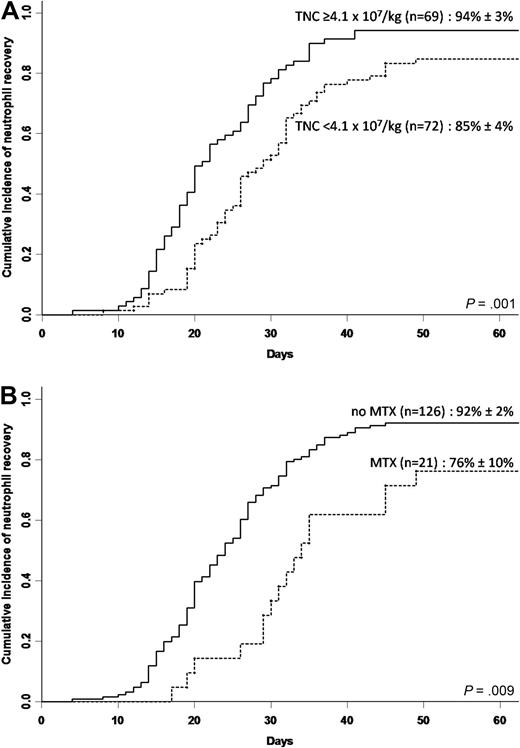
We found that the median infused TNC dose (4.1 × 107/kg) was the best cutoff to separate results of neutrophil engraftment because lower doses failed to stratify results: of 31 patients who had an infused graft with less than 2.7 × 107/kg TNCs (lower quartile), 5 did not achieve neutrophil recovery, and their CI of neutrophil recovery was 84% plus or minus 7%. Three of 18 patients who received an infused TNC dose less than 2.0 × 107/kg, and 1 of 2 patients who received less than 1.0 × 107/kg did not achieve neutrophil recovery. All 36 patients with a median infused TNC dose of at least 6.6 × 107/kg (upper quartile) achieved neutrophil recovery. In univariate analysis (Table 3), CI of neutrophil recovery was higher in patients younger than 5 years (P = .01), in patients receiving an infused TNC dose of at least 4.1 × 107/kg (Figure 1A; P = .001), and in patients not receiving MTX (Figure 1B; P = .009). After multivariate analysis (Table 4), 2 factors remained independently associated with neutrophil recovery: an infused TNC dose of at least 4.1 × 107/kg (P = .003) and the absence of MTX in the GVHD prophylaxis regimen (P < .001). The use of growth factors did not have a significant influence on neutrophil recovery (data not shown).
Univariate analysis for neutrophil recovery, relapse and disease-free survival
| Risk factor . | n . | Neutrophil recovery . | Relapse . | DFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of events . | CI at day 60, % . | P . | No. of events . | CI at 5 y, % . | P . | No. of events . | Prob. at 5 y, % . | P . | ||
| Year of transplantation | ||||||||||
| Before 2000 | 70 | 60 | 84 ± 4 | .07 | 38 | 54 ± 6 | .07 | 46 | 34 ± 6 | .01 |
| 2000 or later | 77 | 73 | 95 ± 3 | 29 | 39 ± 6 | 34 | 54 ± 6 | |||
| Patient sex | ||||||||||
| Female | 57 | 52 | 89 ± 5 | .96 | 22 | 40 ± 7 | .19 | 26 | 53 ± 7 | .11 |
| Male | 90 | 81 | 90 ± 3 | 45 | 51 ± 5 | 54 | 39 ± 5 | |||
| Patient age | ||||||||||
| Younger than 5 y | 73 | 71 | 97 ± 2 | .01 | 36 | 51 ± 6 | .26 | 39 | 45 ± 6 | .87 |
| 5 y or older | 74 | 62 | 82 ± 5 | 31 | 43 ± 6 | 41 | 43 ± 6 | |||
| Disease status at RCBT | ||||||||||
| Early-intermediate | 110 | 101 | 91 ± 3 | .78 | 46 | 43 ± 5 | .03 | 57 | 47 ± 5 | .10 |
| Advanced | 37 | 32 | 86 ± 6 | 21 | 57 ± 8 | 23 | 37 ± 8 | |||
| Infused TNC | ||||||||||
| Less than 4.1 × 107/kg | 72 | 62 | 85 ± 4 | .001 | 34 | 49 ± 6 | .71 | 44 | 37 ± 6 | .11 |
| 4.1 × 107/kg or greater | 69 | 65 | 94 ± 3 | 30 | 45 ± 6 | 33 | 51 ± 6 | |||
| Type of myeloablative conditioning | ||||||||||
| Busulfan-based | 72 | 66 | 92 ± 3 | .33 | 37 | 54 ± 6 | .08 | 41 | 40 ± 6 | .44 |
| TBI-based | 71 | 63 | 88 ± 4 | 28 | 39 ± 6 | 37 | 48 ± 6 | |||
| Use of MTX | ||||||||||
| No | 126 | 117 | 92 ± 2 | .009 | 55 | 45 ± 5 | .15 | 65 | 47 ± 5 | .02 |
| Yes | 21 | 16 | 76 ± 10 | 12 | 57 ± 11 | 15 | 27 ± 10 | |||
| Risk factor . | n . | Neutrophil recovery . | Relapse . | DFS . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of events . | CI at day 60, % . | P . | No. of events . | CI at 5 y, % . | P . | No. of events . | Prob. at 5 y, % . | P . | ||
| Year of transplantation | ||||||||||
| Before 2000 | 70 | 60 | 84 ± 4 | .07 | 38 | 54 ± 6 | .07 | 46 | 34 ± 6 | .01 |
| 2000 or later | 77 | 73 | 95 ± 3 | 29 | 39 ± 6 | 34 | 54 ± 6 | |||
| Patient sex | ||||||||||
| Female | 57 | 52 | 89 ± 5 | .96 | 22 | 40 ± 7 | .19 | 26 | 53 ± 7 | .11 |
| Male | 90 | 81 | 90 ± 3 | 45 | 51 ± 5 | 54 | 39 ± 5 | |||
| Patient age | ||||||||||
| Younger than 5 y | 73 | 71 | 97 ± 2 | .01 | 36 | 51 ± 6 | .26 | 39 | 45 ± 6 | .87 |
| 5 y or older | 74 | 62 | 82 ± 5 | 31 | 43 ± 6 | 41 | 43 ± 6 | |||
| Disease status at RCBT | ||||||||||
| Early-intermediate | 110 | 101 | 91 ± 3 | .78 | 46 | 43 ± 5 | .03 | 57 | 47 ± 5 | .10 |
| Advanced | 37 | 32 | 86 ± 6 | 21 | 57 ± 8 | 23 | 37 ± 8 | |||
| Infused TNC | ||||||||||
| Less than 4.1 × 107/kg | 72 | 62 | 85 ± 4 | .001 | 34 | 49 ± 6 | .71 | 44 | 37 ± 6 | .11 |
| 4.1 × 107/kg or greater | 69 | 65 | 94 ± 3 | 30 | 45 ± 6 | 33 | 51 ± 6 | |||
| Type of myeloablative conditioning | ||||||||||
| Busulfan-based | 72 | 66 | 92 ± 3 | .33 | 37 | 54 ± 6 | .08 | 41 | 40 ± 6 | .44 |
| TBI-based | 71 | 63 | 88 ± 4 | 28 | 39 ± 6 | 37 | 48 ± 6 | |||
| Use of MTX | ||||||||||
| No | 126 | 117 | 92 ± 2 | .009 | 55 | 45 ± 5 | .15 | 65 | 47 ± 5 | .02 |
| Yes | 21 | 16 | 76 ± 10 | 12 | 57 ± 11 | 15 | 27 ± 10 | |||
DFS indicates disease-free survival; OS, overall survival; CI, cumulative incidence; Prob, probability; RCBT, related cord blood transplantation; TNC, total nucleated cells; TBI, total body irradiation; and MTX, methotrexate.
Other factors analyzed but found to have no significant association with any of the outcomes in the whole cohort are patient's cytomegalovirus status, previous autologous transplantation, ABO matching, and sex matching.
Neutrophil recovery. (A) Cumulative incidence of neutrophil recovery according to infused TNC dose in the whole cohort (n = 147). (B) Cumulative incidence of neutrophil recovery according to use of methotrexate (MTX) in the whole cohort (n = 147).
Neutrophil recovery. (A) Cumulative incidence of neutrophil recovery according to infused TNC dose in the whole cohort (n = 147). (B) Cumulative incidence of neutrophil recovery according to use of methotrexate (MTX) in the whole cohort (n = 147).
Multivariate analysis for neutrophil recovery, relapse and disease-free survival
| Factor . | HR . | 95% CI . | P . | Favorable factor . |
|---|---|---|---|---|
| Neutrophil recovery | ||||
| Infused TNC | ||||
| 4.1 × 107/kg or greater | 1.72 | 1.20-2.45 | .003 | Higher infused TNC |
| Less than 4.1 × 107/kg | 1.00 | |||
| Use of MTX | ||||
| Yes | 0.48 | 0.31-0.73 | < .001 | Lack of MTX |
| No | 1.00 | |||
| Relapse | ||||
| Disease status | ||||
| Advanced | 1.81 | 1.04-3.15 | .04 | Early-intermediate disease |
| Early-intermediate | 1.00 | |||
| Disease-free survival | ||||
| Year of transplantation | ||||
| 2000 and later | 0.50 | 0.31-0.79 | .003 | Transplant year ≥ 2000 |
| Before 2000 | 1.00 | |||
| Disease status | ||||
| Advanced | 1.74 | 1.01-2.97 | .04 | Early-intermediate disease |
| Early-intermediate | 1.00 | |||
| Infused TNC | ||||
| 4.1 × 107/kg or greater | 0.56 | 0.34-0.91 | .02 | Higher infused TNC |
| Less than 4.1 × 107/kg | 1.00 |
| Factor . | HR . | 95% CI . | P . | Favorable factor . |
|---|---|---|---|---|
| Neutrophil recovery | ||||
| Infused TNC | ||||
| 4.1 × 107/kg or greater | 1.72 | 1.20-2.45 | .003 | Higher infused TNC |
| Less than 4.1 × 107/kg | 1.00 | |||
| Use of MTX | ||||
| Yes | 0.48 | 0.31-0.73 | < .001 | Lack of MTX |
| No | 1.00 | |||
| Relapse | ||||
| Disease status | ||||
| Advanced | 1.81 | 1.04-3.15 | .04 | Early-intermediate disease |
| Early-intermediate | 1.00 | |||
| Disease-free survival | ||||
| Year of transplantation | ||||
| 2000 and later | 0.50 | 0.31-0.79 | .003 | Transplant year ≥ 2000 |
| Before 2000 | 1.00 | |||
| Disease status | ||||
| Advanced | 1.74 | 1.01-2.97 | .04 | Early-intermediate disease |
| Early-intermediate | 1.00 | |||
| Infused TNC | ||||
| 4.1 × 107/kg or greater | 0.56 | 0.34-0.91 | .02 | Higher infused TNC |
| Less than 4.1 × 107/kg | 1.00 |
HR indicates hazard ratio; CI, confidence interval; TNC, total nucleated cell dose; MTX, methotrexate
Variables included in the multivariate analysis models for all cases were (1) for neutrophil recovery: year of transplantation (before 2000 vs 2000 or later), patient's age (<5 vs ≥5 years), disease status (early-intermediate vs advanced), infused TNC (<4.1 vs ≥4.1 times] 07/kg), and use of MTX (no vs yes); (2) for relapse: year of transplantation (before 2000 vs 2000 or later), disease status (early-intermediate vs advanced), and use of TBI (no vs yes); and (3) for disease-free survival: year of transplantation (before 2000 vs 2000 or later), sex (female vs male), disease status (early-intermediate vs advanced), infused cell dose (<4.1 vs ≥4.1 × 107/kg), and use of MTX (no vs yes).
One hundred twenty-two patients reached a platelet count of at least 20 × 109/L at a median of 38 days (range, 11-253 days). The CI of platelet recovery at day +180 was 84% plus or minus 3%.
Graft-versus-host disease
Grade II to IV acute GVHD CI at day +100 was 12% plus or minus 3%, with a median of 12.5 days to the onset of acute GVHD after CBT (range, 8-25 days). The severity of acute GVHD was low with 34 patients having grade I, 16 with grade II, 1 with grade III, and none with grade IV. Fourteen patients developed chronic GVHD, only 2 of extensive grade, after a median of 236 days after CBT (range, 85-473 days). CI of chronic GVHD at 2 years was 10% plus or minus 2%. Given the low incidence of both acute and chronic GVHD, a risk factor analysis for these outcomes was not performed.
Nonrelapse causes of death and nonrelapse mortality
Fifteen patients (23%) died of transplantation-related causes, including 6 patients from infection and 2 patients after second transplantation with bone marrow from the CB donor, performed for relapse of leukemia (one with CML in first chronic phase dying of hemorrhage and the other with ALL in second CR dying of bronchiolitis obliterans). Nonrelapse deaths after initial RCBT (n = 13, as reported in Table 5) occurred early, at a median of 25 days (range, 5 days to 2.6 years). CI of NRM at 5 years was 9% plus or minus 2%. A risk factor analysis was not performed, given the low number of events.
Nonrelapse deaths, relapse, response to DLI, and second transplantations according to diagnosis and disease status at RCBT
| Remission or phase/disease status at RCBT . | N . | Nonrelapse deaths, n . | Relapse, n . | Median time from RCBT to relapse, mo (range) . | DLI . | Second transplantations* . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N . | Response, n† . | Median duration of response, y (range)† . | N . | Response, n† . | Median duration of response, y (range)† . | |||||
| Acute leukemia | ||||||||||
| First CR/early | 31 | 3 | 10 | 8.0 (1.0-27.8) | 1 | 1 | 15.7 | 4 | 4 | 4.0 (1.1-8.7) |
| Second CR/int | 51 | 4 | 23 | 8.7 (1.5-17.5) | 4 | 1 | 1.8 | 7‡ | 2 | 5.4 (2.5-8.3) |
| Third or more CR/int | 13 | 4 | 5 | 4.6 (1.0-13.5) | 0 | NA | NA | 2 | 0 | NA |
| Refractory/advanced | 14 | 2 | 9 | 3.2 (0.4-7.9) | 1 | 0 | NA | 0 | NA | NA |
| MDS Advanced | 17 | 0 | 8 | 3.7 (0.6-26.0) | 1 | 0 | NA | 5‡ | 2 | 5.0 (3.0-6.9) |
| CML | ||||||||||
| First chronic phase/early | 9 | 0 | 6 | 11.9 (4.9-43.7) | 2 | 1 | 13.4 | 2 | 0 | NA |
| Accelerated phase/int | 3 | 0 | 2 | 28.5 (9.9-47.1) | 2 | 1 | 5.3 | 1‡ | 1 | 9.2 |
| Lymphoma, second or more CR/int | 3 | 0 | 0 | NA | 0 | NA | NA | 0 | NA | NA |
| Solid tumors, advanced | 6 | 0 | 4 | 7.5 (5.6-9.7) | 0 | NA | NA | 0 | NA | NA |
| Total | 147 | 13 | 67 | 6.8 (0.4-47.1) | 11 | 4 (36%) | 9.2 (1.8-15.7) | 21 | 9 (43%) | 6.0 (1.1-9.2) |
| Remission or phase/disease status at RCBT . | N . | Nonrelapse deaths, n . | Relapse, n . | Median time from RCBT to relapse, mo (range) . | DLI . | Second transplantations* . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| N . | Response, n† . | Median duration of response, y (range)† . | N . | Response, n† . | Median duration of response, y (range)† . | |||||
| Acute leukemia | ||||||||||
| First CR/early | 31 | 3 | 10 | 8.0 (1.0-27.8) | 1 | 1 | 15.7 | 4 | 4 | 4.0 (1.1-8.7) |
| Second CR/int | 51 | 4 | 23 | 8.7 (1.5-17.5) | 4 | 1 | 1.8 | 7‡ | 2 | 5.4 (2.5-8.3) |
| Third or more CR/int | 13 | 4 | 5 | 4.6 (1.0-13.5) | 0 | NA | NA | 2 | 0 | NA |
| Refractory/advanced | 14 | 2 | 9 | 3.2 (0.4-7.9) | 1 | 0 | NA | 0 | NA | NA |
| MDS Advanced | 17 | 0 | 8 | 3.7 (0.6-26.0) | 1 | 0 | NA | 5‡ | 2 | 5.0 (3.0-6.9) |
| CML | ||||||||||
| First chronic phase/early | 9 | 0 | 6 | 11.9 (4.9-43.7) | 2 | 1 | 13.4 | 2 | 0 | NA |
| Accelerated phase/int | 3 | 0 | 2 | 28.5 (9.9-47.1) | 2 | 1 | 5.3 | 1‡ | 1 | 9.2 |
| Lymphoma, second or more CR/int | 3 | 0 | 0 | NA | 0 | NA | NA | 0 | NA | NA |
| Solid tumors, advanced | 6 | 0 | 4 | 7.5 (5.6-9.7) | 0 | NA | NA | 0 | NA | NA |
| Total | 147 | 13 | 67 | 6.8 (0.4-47.1) | 11 | 4 (36%) | 9.2 (1.8-15.7) | 21 | 9 (43%) | 6.0 (1.1-9.2) |
RCBT indicates related cord blood transplantation; DLI, donor lymphocyte infusion; CR, complete remission; MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; int: intermediate.
Donor types: 15 with bone marrow (n = 14) or peripheral blood stem cells (n = 1) from the same donor; 1 haploidentical stem cell transplantation from the mother (acute myeloid leukemia in first CR), 3 unrelated bone marrow (1 acute lymphoblastic leukemia [ALL] in second CR, 1 MDS, 1 CML in accelerated phase), and 2 unspecified donor types (1 ALL in first CR, 1 ALL in third or more CR).
Response to DLI or second transplantation was defined as no relapse or death occurring after treatment until the date of last follow-up.
A total of 3 patients (one with acute leukemia, one with MDS, and one with CML) received a second transplantation after failure of DLI.
Relapse
Relapse occurred in 67 patients within a median time of 6.8 months (range, 11 days to 3.9 years) after RCBT. Overall, CIs of relapse were 35% plus or minus 4% at 1 year, 43% plus or minus 4% at 2 years, and 47% plus or minus 4% at 5 years. Early or intermediate disease was the only factor found to significantly decrease the 5-year CIs of relapse in both univariate (Table 3; P = .03) and multivariate analysis (Table 4; P = .04).
Among the 109 patients with AL, CIs of relapse were 35% plus or minus 5% at 1 year, 43% plus or minus 5% at 2 years, and 44% plus or minus 5% at 5 years. There were 48 relapses: 10 of 31 patients in first CR (CI at 5 years, 33% ± 9%), 23 of 51 patients in second CR (CI at 5 years, 46% ± 7%), 5 of 13 patients in third or subsequent CR (CI at 5 years, 39% ± 14%), and 9 of 14 patients with refractory disease (CI at 5 years, 64% ± 14%; P = .04). Univariate analysis showed that a lower relapse incidence at 5 years was seen in patients given RCBT in remission compared with patients not in remission (41% ± 5% vs 64% ± 14%; P = .007) and in patients receiving a myeloablative conditioning regimen with TBI compared with busulfan (35% ± 6% vs 55% ± 8%; P = .03). No statistical difference was observed in relapse incidence between ALL and acute myeloid leukemia cases. After multivariate analysis adjusted for patient's age and sex, a state of CR at time of transplantation (hazard ratio, 0.40; 95% CI, 0.17-0.96; P = .04) and the use of TBI (hazard ratio, 0.52; 95% CI, 0.29-0.94; P = .03) remained independently associated with a lower relapse incidence in patients with AL.
Treatment of relapse
Treatment for relapse was reported for 59 of 67 relapsing patients. Ten patients (17%) received supportive care, all dying within a median of 45 days (range, 3 days to 24 months) after relapse. Chemotherapy was administered to 33 patients, including 20 patients (34%) for whom this was the only treatment given for relapse. Of those 20 patients, only 2 are alive at last follow-up (one with ALL in first CR and one with CML in first chronic phase given chemotherapy and subsequently imatinib mesylate), the other 18 patients died at a median of 12 months (range, 6 days to 33 months) after relapse. Treatment of relapse was not reported for 8 patients: 7 of them died at a median of 2.0 months (range, 0.6-8.3 months) after relapse and 1 died at 43.6 months after relapse. Eleven patients received at least one DLI from the initial CB donor, administered at a median of 11.0 months (range, 3.6 months to 4.5 years) after CBT. Second transplantation was performed in 21 relapsing patients (including 3 patients who also received DLI) at a median of 12.4 months after CBT (range, 4.8-38.0 months). Fifteen second transplantations were done with bone marrow (n = 14) or peripheral blood stem cells (n = 1) from the same RCB transplant donor; 1 patient was given a haplo-identical stem cell transplant from the mother, and 3 patients underwent an unrelated BMT. In 2 cases, the second transplant donor was not specified. Details on the response to DLI and second transplantations according to diagnosis and disease status are given in Table 5.
Disease-free survival
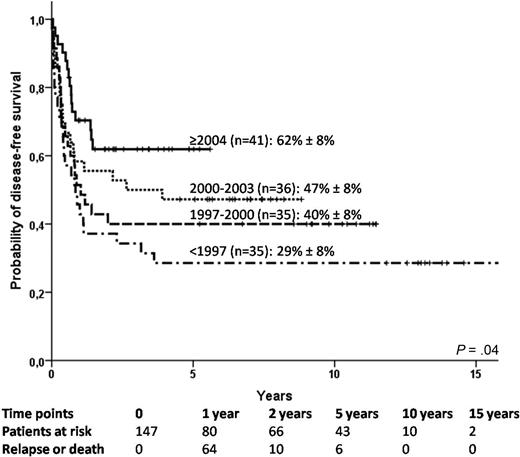
The probability of DFS was 56% plus or minus 4% at 1 year, 49% plus or minus 4% at 2 years, and 44% plus or minus 4% at 5 years. DFS was found to improve over time (Figure 2; P = .04). Indeed, there was a strong association between the use of MTX and the year of transplantation: 29% patients received MTX before 1997, 26% from 1997 to 1999, 6% from 2000 to 2003, and none since 2004 (P < .001). In the AL population, the proportion of patients who received a transplant in first or second CR increased overtime: 54% before 1997, 84% from 1997 to 1999, 72% from 2000 to 2003, and 90% since 2004 (P = .01). Table 3 shows the univariate analysis for DFS. In multivariate analysis, transplantation year of 2000 or later (P = .003), early or intermediate disease (P = .04), and infused TNCs of at least 4.1 × 107/kg (P = .02) were found to predict better DFS (Table 4).
Disease-free survival according to year of transplantation in the whole cohort (n = 147).
Disease-free survival according to year of transplantation in the whole cohort (n = 147).
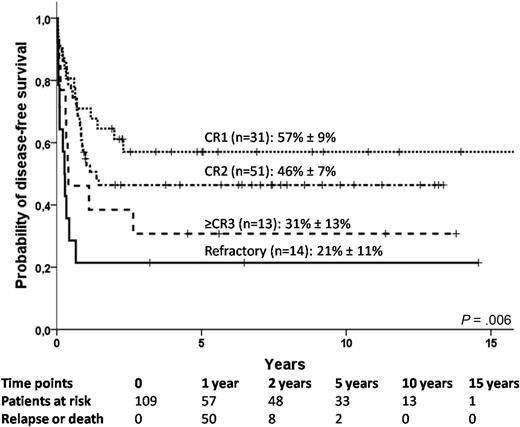
For the subgroup of patients with AL, 5-year DFS was influenced by remission status, because it was 57% plus or minus 9%, 46% plus or minus 7%, 31% plus or minus 13%, and 21% plus or minus 11%, respectively, for patients who received a transplant in first CR, second CR, third CR, and in refractory disease, (Figure 3; P = .006). The 5-year DFS of patients who did or did not receive MTX was 24% plus or minus 10% and 48% plus or minus 5%, respectively (P = .007). After multivariate analysis adjusted for year of transplantation, patient's sex and use of TBI, only a state of CR (hazard ratio, 0.41; 95% CI, 0.21-0.79; P = .008), and the lack of MTX in GVHD prophylaxis regimen (hazard ratio, 0.48; 95% CI, 0.26-0.89; P = .02) remained significantly associated with superior DFS.
Disease-free survival in acute leukemia patients according to remission status (n = 109).
Disease-free survival in acute leukemia patients according to remission status (n = 109).
Overall survival
The probability of OS was 75% plus or minus 4% at 1 year, 63% plus or minus 4% at 2 years, and 55% plus or minus 4% at 5 years. Similarly to DFS, OS was also found to improve over time with an OS at 5 years of 34% plus or minus 8% before 1997, 55% plus or minus 6% from 1997 to 2003, and 76% plus or minus 7% since 2004 (P = .003). Patients not receiving MTX had improved survival compared with patients who received this drug for GVHD prophylaxis (58% ± 5% vs 33% ± 10%; P = .006). Patients with early disease had a better survival (74% ± 7%) than patients with intermediate (51% ± 6%) or advanced disease (42% ± 8%; P = .02). After multivariate analysis adjusted for infused cell dose, year of transplantation of at least 1997 (hazard ratio, 0.54; 95% CI, 0.32-0.91; P = .02), early disease (hazard ratio, 0.44; 95% CI, 0.23-0.87; P = .02), and absence of methotrexate (hazard ratio, 0.54; 95% CI, 0.29-0.99; P = .05) were found to be independently associated with better OS. In patients with AL, OS was 71% plus or minus 4% at 1 year, 61% plus or minus 5% at 2 years, and 51% plus or minus 5% at 5 years.
Discussion
In this registry-based study, we identified 3 main factors that influenced outcomes after HLA-identical RCBT in malignancies: cell dose, use of MTX for GVHD prophylaxis, and disease status at transplantation. A higher infused TNC dose, using the median (≥ 4.1 × 107/kg) to separate groups, was associated with better neutrophil recovery. Lower TNC dose cutoffs did not stratify neutrophil engraftment rates any further. This is the first report to show an influence of cell dose on neutrophil recovery in the HLA-identical RCBT setting, and this finding may thus warrant confirmation in a larger cohort of patients. Indeed, cell dose has been identified as a predictor of engraftment in the unrelated HLA-mismatched CBT setting5,12-15 and is therefore considered to be a major criterion for CB unit selection, together with HLA matching. Three of 18 patients who received less than 2.0 × 107/kg TNCs in this study failed to engraft. An option when collected cell dose is low in the related setting could be to add bone marrow cells from the same sibling donor, although, to our knowledge, there are no published reports of this practice.
As previously reported in patients with hemoglobinopathies3 and in a more limited cohort of patients with malignant diseases undergoing RCBT,4 the use of MTX for GVHD prophylaxis was shown to have a negative effect on neutrophil recovery. MTX effectively prevents GVHD; however, in view of the low incidence of this complication after CBT (particularly after HLA-identical RCBT) and of the negative effect of the drug on neutrophil recovery and on the graft-versus-tumor effect, the use of MTX appears detrimental after RCBT. Indeed, inclusion of MTX in GVHD prophylaxis has considerably decreased over time, because none of the patients included in this analysis and undergoing transplantation after 2002 received this drug. Finally, patients undergoing transplantation with early disease had better DFS, particularly in the subgroup of patients with AL whereby remission status was strongly associated with DFS. This influence of disease status on relapse, DFS, and OS has been well described in previous reports of related and unrelated CBT for malignancies.5,7,15,16 We did not observe an influence of the recipient's cytomegalovirus serologic status, as well as of the recipient's age on the different outcomes after adjusting for other factors.
Survival rates have improved over time. This can be explained by the decreasing use of MTX, as well as by better patient selection over time, particularly for AL cases whereby the proportion of patients undergoing transplantation in first and second remission was higher in more recent years. Other factors not evaluated in this study that may have also played a role in this improvement are the growing experience with CBT and better treatment of infections and other transplantation-related complications.
As previously reported in RCBT,2-4 we found a low incidence of NRM, as well as low incidence and severity of acute and chronic GVHD. Indeed, incidences of NRM and GVHD increase with increasing HLA disparities between CB donor and recipient, both in the related and the unrelated CBT setting.5,7,17 Given that the CB donors in this cohort were all HLA-identical relatives, this explains these results.
Relapse incidence was relatively high in the present study, being 43% at 2 years and 47% at 5 years. A relapse rate of 20% at 2 years was reported in the Cord Blood Transplantation study17 that included 191 children with hematologic malignancies undergoing unrelated CBT. The difference in relapse rates between related and unrelated CBT probably reflects a stronger graft-versus-tumor effect after unrelated CBT, given that in the unrelated setting most CB units are HLA-mismatched. However, in the Cord Blood Transplantation study, a high NRM rate compensated for the low relapse rate and resulted in an OS of 50% at 2 years,17 similar to what is seen in the present study.
A comparative study between HLA-identical sibling CBT and BMT previously showed no difference in OS despite an initial lower engraftment rate in the CBT group.2 Incidences of acute and chronic GVHD were lower in the CBT group. Neither relapse nor DFS were compared because follow-up was short. In the present study, we found a probability of DFS of 44% at 5 years (53% for patients receiving transplants since 2000) in patients with AL and a strong association with remission status. In the few studies of related HLA-identical BMT for AL in pediatric patients, DFS at 5 years varied from 40% to 60%.18,19 One advantage of a related donor is the availability of this donor for a second transplantation or DLI if required. In fact, in our study, 25 of the 59 relapsing patients for whom treatment of relapse was reported received either DLI or BMT from the initial CB donor with a response rate of 36% and 40% for DLI and BMT, respectively.
Until recently, and still today in most countries, RCBTs have relied on collaboration between highly motivated families, obstetricians, midwifes, and transplantation center hematologists. In the past years, several directed sibling CB banks have been established in different countries.20-23 Experience has, however, shown that the utilization rate of stored related CB units is low, particularly in the setting of malignant diseases (Ferry et al, unpublished data from 2 such banks in Paris, 2009). Whether directed sibling CB banks should be implemented on a large scale for clinical use in patients with malignancies would require discussions with considerations for the indications for banking, costs, and effectiveness.
All CB donors were younger siblings (and one patient's offspring), and the patient's diagnosis antedated the CB donor's birth. In 60 cases, the mother was pregnant at the time of diagnosis, and the CB unit was stored or used immediately after birth. Interestingly, in 82 cases, the CB donor was conceived after diagnosis. Whether this was done with the aim of conceiving a “saviour sibling” or simply a reflection of the fact that a significant proportion of parents of a young child with a malignancy may still be planning to expand their family is unknown. Preimplantation genetic diagnosis with HLA-typing has been used to treat patients with nonmalignant inherited disorders,24-26 but, to our knowledge; there is no report of this technique being applied in the setting of a malignant disorder.
In summary, cell dose, use of MTX, and disease status are the main factors found to influence outcomes after HLA-identical RCBT. The good outcomes presented here support banking and use of HLA-identical sibling CB. If the mother of a child with a hematologic malignancy who may require transplantation becomes pregnant, all efforts should be made to cryopreserve the CB of the newborn sibling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.-L.H., N.K., P.T., E.G., and V.R. designed the study; A.-L.H., N.K., P.T., W.C., and I.I. contacted centers and collected data; C.M.S.B., F.L., K.T., A.L., J.-P.J., C.M., Y.B., C.D.d.H., and S.K.N. submitted at least 4 patients/center for the study; A.-L.H., I.I., and V.R. analyzed data; A.-L.H. and V.R. wrote the paper; and A.-L.H., N.K., C.M.S.B., P.T., F.L., K.T., A.L., J.-P.J., C.M., Y.B., C.D.d.H., C.P., W.C., S.K.N., I.I., E.G., and V.R. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of Eurocord-EBMT members appears as an online data supplement to this article (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Andrée-Laure Herr, Eurocord, Hôpital Saint-Louis, 1 ave Claude Vellefaux, Quadrilatère porte 5, 75475 Paris cedex 10, France; e-mail: andree-laure.herr-bellon@sls.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal