Dysregulated cholesterol transport and metabolism are important risk factors for atherothrombosis and are commonly associated with enhanced platelet reactivity; however, the molecular mechanisms linking hypercholesterolemia with increased platelet function remain unclear. In this issue of Blood, Ma et al define an important role for the platelet lipoprotein scavenger receptor SR-BI in regulating platelet hyperactivity and thrombosis in hyperlipidemic states.1
The exaggerated accumulation of platelets at sites of atherosclerotic plaque rupture is an important step in the development of arterial thrombi, and is the principal pathogenic mechanism underlying acute myocardial infarction and ischemic stroke. Heightened platelet reactivity is an important risk factor for arterial thrombosis and is commonly observed in patients with diabetes and hyperlipidemia. The mechanisms underlying increased platelet reactivity in hyperlipidemic states are complex and diverse, including the direct platelet-activating effects of oxidized low-density lipoprotein (LDL), changes in the intrinsic reactivity of platelets due to cholesterol loading of platelet membranes, as well as oxidative modification of membrane lipids and glycoproteins.2-4 In a previous study, Podrez and colleagues demonstrated an important link between oxidative stress, dyslipidemia (increased serum LDL/triglycerides and decreased high-density lipoprotein [HDL]), and enhanced platelet reactivity.4 These authors demonstrated that pathophysiologic plasma levels of oxidized choline glycerophospholipids (termed oxPCCD36) stimulate platelet activation through the lipid scavenger receptor, CD36. In the current study, Ma et al demonstrate an important role for a second scavenger receptor, SR-BI, in regulating platelet hyperactivity in the context of hyperlipidemia.
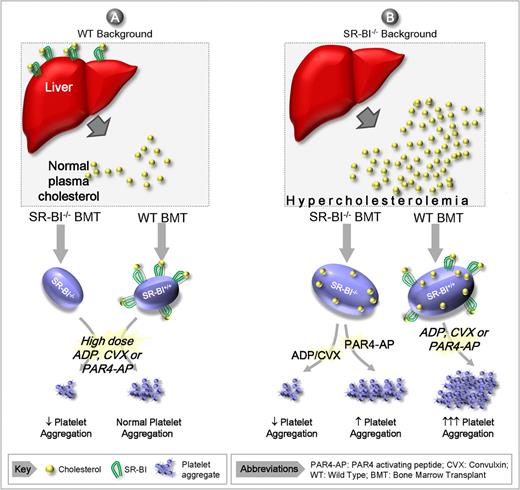
Scavenger receptor-BI plays a pivotal role in cholesterol metabolism. SR-BI is a multiligand receptor member of the CD36 superfamily. SR-BI functions to scavenge cholesterol esters from HDLs in tissues such as the liver for use in steroidogenesis.5 Thus, the loss of SR-BI expression in such tissues results in excessive accumulation of lipoproteins in the circulation (see figure). The dyslipidemia associated with SR-BI deficiency is associated with increased unesterified cholesterol in the circulation as well as increased cholesterol loading in platelet membranes. Platelets have also recently been shown to express SR-BI on their surface; however, the role of this receptor in platelet function has not been well defined.
Proposed model for the role of SR-BI in modulating platelet reactivity. (A) Normolipidemia. Under normolipidemic conditions the platelet count and cholesterol content remain normal irrespective of whether platelets express SR-BI. Note that SR-BI/− platelets have reduced responsiveness to high concentrations of soluble agonists relative to wild-type (WT) controls. (B) Hyperlipidemia. In the absence of SR-BI expression by steroidogenic tissues (principally the liver), there is reduced cholesterol uptake from the circulation, leading to hyperlipidemia. The severe hyperlipidemia in SR-BI−/− results in thrombocytopenia and an increased platelet cholesterol content irrespective of whether platelets express SR-BI. Under hyperlipidemic conditions, SR-BI−/− platelets have increased responsiveness to PAR4 agonists, but a paradoxical hyporesponsive to other agonists. Note that WT platelets are hyperresponsive to all agonists in the presence of hyperlipidemia.
Proposed model for the role of SR-BI in modulating platelet reactivity. (A) Normolipidemia. Under normolipidemic conditions the platelet count and cholesterol content remain normal irrespective of whether platelets express SR-BI. Note that SR-BI/− platelets have reduced responsiveness to high concentrations of soluble agonists relative to wild-type (WT) controls. (B) Hyperlipidemia. In the absence of SR-BI expression by steroidogenic tissues (principally the liver), there is reduced cholesterol uptake from the circulation, leading to hyperlipidemia. The severe hyperlipidemia in SR-BI−/− results in thrombocytopenia and an increased platelet cholesterol content irrespective of whether platelets express SR-BI. Under hyperlipidemic conditions, SR-BI−/− platelets have increased responsiveness to PAR4 agonists, but a paradoxical hyporesponsive to other agonists. Note that WT platelets are hyperresponsive to all agonists in the presence of hyperlipidemia.
Using a series of mouse knockout and bone marrow transplantation models, Ma et al investigated the effects of SR-BI deficiency on platelet aggregation and thrombosis. Using platelets isolated from SR-BI−/− mice, they demonstrated differential responsiveness to adenosine diphosphate (ADP), protease-activated receptor 4 (PAR4) activating peptide, and the GPVI ligand, convulxin. SR-BI−/− platelets had a heightened response to PAR4-activating peptides, yet a blunted response to ADP and convulxin. This partial defect in platelet responsiveness to ADP and convulxin was not entirely unexpected, as SR-BI−/− mice are thrombocytopenic and their platelets have an altered morphology. Notably, this defect in platelet morphology and function is not due to platelet SR-BI deficiency per se, as SR-BI−/− mice transplanted with wild-type bone marrow (ie, platelets expressing normal levels of SR-BI) also exhibit a moderate thrombocytopenia and altered morphology. These findings suggest that the hyperlipidemic milieu associated with non–bone marrow–derived SR-BI deficiency is the principal cause of the thrombocytopenia and abnormal platelet morphology.
Using bone marrow reconstitution models, the authors elegantly demonstrate a dramatic shift in platelet reactivity as a function of SR-BI expression in bone marrow and non–bone marrow tissues. Their studies demonstrate that non–bone marrow SR-BI deficiency (presumably SR-BI in the liver) is the principal cause of platelet hyperreactivity, due to a profound increase in serum lipoproteins and a marked increase in platelet cholesterol content. However, enhanced platelet reactivity to all agonists was only observed with SR-BI expression in platelets. Thus, there appear to be 2 requirements for global platelet hyperactivity in SR-BI–deficient mice: (1) a marked dyslipidemia due to loss of the cholesterol scavenging function of SR-BI in non–marrow tissue and (2) unperturbed platelet expression of SR-BI, leading to increased platelet reactivity.
Ma et al demonstrate that SR-BI deficiency alone, under normolipidemic conditions, does not lead to a major defect in platelet aggregation, suggesting that this receptor is unlikely to play a major role in regulating the hemostatic function of platelets. In contrast, the authors convincingly demonstrate that platelet-expressed SR-BI plays an important role in promoting thrombosis in several distinct models of dyslipidemia, raising the interesting possibility that selectively targeting platelet SR-BI may represent a safe and effective approach to reduce platelet hyperactivity in dyslipidemic states.
These new findings by Ma et al provide further insight into the complex interplay between hyperlipidemia, platelet hyperreactivity, and a prothrombotic phenotype. Putative mechanisms for the modulation of platelet function by SR-BI have been proposed, including alterations in cholesterol organization required for the assembly of lipid rafts. However, the demonstration that the SR-BI receptor itself has an intrinsic role in modulating platelet responses to higher concentrations of physiologic agonists suggests that other mechanisms may also be involved. Future studies will be required to more clearly define the molecular mechanisms by which SR-BI modulates platelet function. In addition, a clearer understanding of the relationship between the prothrombotic effects of specific oxidized phospholipids through CD36 with the altered cholesterol loading through SR-BI are required to fully understand the approaches that are likely to be most effective at reducing increased platelet reactivity in dyslipidemic states.
Although these recent studies shed new light on the role of scavenger receptors in regulating platelet function, their importance to platelet hyperreactivity in humans remains to be determined as the mouse models do not accurately reflect the dyslipidemia that occurs in humans. Nonetheless, these recent studies by Ma et al offer potential new insights into the refractoriness of conventional antiplatelet therapies often encountered in disease states that accompany hyperlipidemia such as type II diabetes and the metabolic syndrome. Given the importance of CD36 and SR-BI in lipid scavenging in a range of cell types, the challenge remains to develop strategies that reduce the prothrombotic effects of plasma lipoproteins while minimizing the impact of these therapies on cholesterol uptake and steroidogenesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■
REFERENCES
Author notes
Contribution: Z.S.K. and S.P.J. wrote manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal