In this issue of Blood, Lin and colleagues address in detail the nature and severity of telomere disruption in chronic lymphocytic leukemia (CLL).1 Using sophisticated methods for telomere length determination, the authors show that critical telomere shortening occurs in a proportion of CLL samples and correlates with the presence of large-scale genomic rearrangements occurring in telomeric regions. These findings suggest that genomic instability associated with severe telomere shortening contributes to the progression of CLL and might represent a fairly common mechanism of tumor progression occurring in several human cancers.
There is increasing evidence linking telomere biology and cancer development. Yet the role of telomeres and telomere regulatory machinery in tumorigenesis remains elusive and often seems paradoxical. Due to their progressive shortening in response to proliferative and oxidative stress, telomeres are regarded as proliferative checkpoints and yet severe telomeric shortening has been associated with tumor development and progression. Telomerase, a critical regulator of telomere length (TL), has oncogene-like properties; however, its absence or hypofunction is associated with increased risk of tumor development. These contradictory findings can be explained by the natural history of cells undergoing progressive telomeric attrition. Once progressive telomere shortening crosses a specific threshold, cells enter senescence and undergo proliferative arrest acting as important tumor suppressor mechanisms. However, in vitro studies indicate that if the senescence checkpoint is released, cells continue to proliferate while undergoing further telomeric shortening, leading to a condition known as crisis that is associated with severe genomic instability and increased risk of transformation.2 Most tumors have telomeres that are shorter than their normal counterparts and TL has been associated with a worse outcome in many common human cancers.3 Nevertheless, there has been no clear demonstration that telomeres could shorten in vivo up to the level of inducing crisis-like phenomena. Moreover, a direct link between severe telomere attrition, genomic instability, and tumor progression so far has not been established in primary human cancer cells.
CLL is one of the hematologic tumors in which telomere dynamics have been most extensively investigated. Early studies emphasized the association between TL and VH-mutational status.4,5 Later studies have validated the prognostic value of TL for several outcome variables and established its independent prognostic role in addition to the better-established predictors such as cytogenetics, flow cytometry, and VH-mutational status.6 Despite the strong correlation with outcome, few studies have investigated the link between telomere dynamics and CLL progression. Poncet et al have evaluated the expression of telomere-related proteins and found a global deregulation of the telomeric protein machinery with reduced expression of hTERT, DYSKERIN, TRF1, hRAP1, POT1, hEST1A, MRE11, RAD50, and KU80, and increased expression of TPP1 and RPA1.7 Finally, a recent report has documented an increased incidence of CLL in subjects who are heterozygotes for hypofunctional variants of telomerase.8
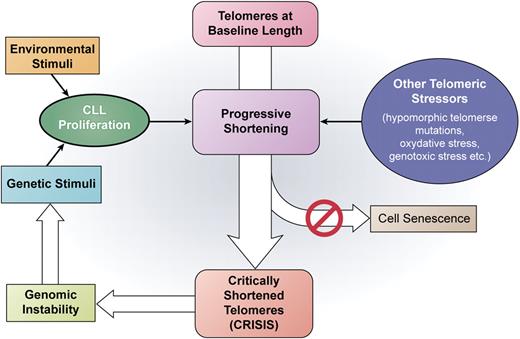
The work from Lin et al provides evidence supporting a direct link between telomere deregulation and CLL progression. In their report, Lin and colleagues investigated the presence of dysfunctional telomeres in CLL using a novel approach known as single-telomere length analysis (STELA).1 The high resolution capable with STELA allowed the full extent of telomeric defects in CLL to be established, demonstrating that TL in patients with poor prognosis was comparable with that observed in cells undergoing crisis in vitro: this phase of cell life is known to be associated with an extremely high level of genomic instability. Furthermore, the authors report that the presence of critically shortened telomeres was associated with severe genomic instability as shown by the presence of complete telomeric loss events, fusion events, and genomic rearrangements concentrated in telomeric regions. The presence of dysfunctional telomeres was more common but not exclusively found in patients with poor prognostic features (Binet stage C, VH-unmutated status, and high Zap-70 expression), suggesting that a proportion of patients might show evidence of telomeric dysfunction even at early disease stages. Taken together, these results provide convincing evidence that crisis-like phenomena associated with dysfunctional telomeres play a role in CLL progression (see figure) and might be involved in critical events such as chromosome 17p loss. Finally, as severe telomere attrition is not found exclusively in CLL but rather occurs in many hematologic and solid tumors, a reasonable hypothesis can be made that tumor progression associated with critical telomere shortening might also be of relevance in other tumors.
A schematic representation of the role of progressive telomere shortening in driving CLL progression based on current knowledge, with a main focus on the results from Lin et al in this issue.1 CLL proliferation is induced by either genetic or environmental stimuli. Repeated courses of cell division as well as other telomeric stressors induce progressive telomere shortening. Due to the inactivation of the senescence checkpoint, cells with critically shortened telomeres enter a crisis-like condition that leads to genomic instability allowing further clonal evolution and disease progression. Professional illustration by Kenneth X. Probst.
A schematic representation of the role of progressive telomere shortening in driving CLL progression based on current knowledge, with a main focus on the results from Lin et al in this issue.1 CLL proliferation is induced by either genetic or environmental stimuli. Repeated courses of cell division as well as other telomeric stressors induce progressive telomere shortening. Due to the inactivation of the senescence checkpoint, cells with critically shortened telomeres enter a crisis-like condition that leads to genomic instability allowing further clonal evolution and disease progression. Professional illustration by Kenneth X. Probst.
The report has a few limitations, in particular the small number of patients studied and lack of strong clinical correlations. An association with poor prognostic features was observed but the impact of TL determination by STELA on survival, treatment requirement, drug sensitivity, and so on still needs to be addressed in large cohorts of CLL patients with adequate follow-up. As TL proved of high prognostic value even when measured with less sophisticated tools, I expect to find these correlations highly positive with STELA also. From a more speculative point of view, the ability of CLL cells to actually bypass the senescence checkpoint and enter crisis in the absence of permitting lesions such as, for example, p53 loss or chromosome 11 deletion—both quite uncommon events in early CLL—is not fully understood. Clearly, the impact of a telomere-related progression mechanism would be broader if proven to occur even in the absence of these major genetic defects.
Despite these limitations, this report establishes a clear link between telomere deregulation, genomic instability, and CLL progression. Future studies are required to address the relative impact of different stressors on telomere dynamics (including proliferation drive, specific genetic lesions, microenvironment, host genetic background, treatment, and so on) that might explain the large variability of TL in CLL patients. From a more general point of view, it will be fascinating to see if the same mechanisms come into play in other human cancers.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■