Abstract
Cartilage-hair hypoplasia (CHH) is a rare autosomal recessive disease caused by mutations in the RMRP gene. Beside dwarfism, CHH has a wide spectrum of clinical manifestations including variable grades of combined immunodeficiency, autoimmune complications, and malignancies. Previous reports in single CHH patients with significant immunodeficiencies have demonstrated that allogeneic hematopoietic stem cell transplantation (HSCT) is an effective treatment for the severe immunodeficiency, while growth failure remains unaffected. Because long-term experience in larger cohorts of CHH patients after HSCT is currently unreported, we performed a European collaborative survey reporting on 16 patients with CHH and immunodeficiency who underwent HSCT. Immune dysregulation, lymphoid malignancy, and autoimmunity were important features in this cohort. Thirteen patients were transplanted in early childhood (∼ 2.5 years). The other 3 patients were transplanted at adolescent age. Of 16 patients, 10 (62.5%) were long-term survivors, with a median follow-up of 7 years. T-lymphocyte numbers and function have normalized, and autoimmunity has resolved in all survivors. HSCT should be considered in CHH patients with severe immunodeficiency/autoimmunity, before the development of severe infections, major organ damage, or malignancy might jeopardize the outcome of HSCT and the quality of life in these patients.
Introduction
Cartilage-hair hypoplasia (CHH) is a rare form of autosomal recessive dwarfism, first described in the Old Order Amish population of Pennsylvania.1 CHH has a wide spectrum of clinical characteristics including: prenatal onset of growth failure, metaphyseal chrondrodysplasia, fine and sparse hair, immunodeficiency, anemia, Hirschsprung disease, orthopedic problems, and increased risk of malignancies. Incidence is estimated at 1.5 in 1000 births in the Amish and 1 in 23 000 in the Finnish population.2-5
CHH is caused by mutations of the RMRP gene, that maps on chromosome 9p21-p12 and encodes for the RNA component of the mitochondrial RNA processing (MRP) endoribonuclease. This RNA-cleaving enzyme encodes for structural RNA and plays a role in nuclear DNA replication and cell growth. Recent data indicate that RMRP forms a ribonucleoprotein complex with the telomerase reverse transcriptase (TERT) catalytic subunit. This complex has RNA polymerase activity and produces dsRNAs that can be processed to small-interfering RNAs in a Dicer-dependent manner.6 In CHH patients, mutations of this gene have been shown to interfere with the expression of RMRP transcript and may affect a variety of biologic processes.7-14
The immunodeficiency in patients with CHH is very variable, but more than 30% have significant CD4+ and CD8+ lymphocytopenia. Normal in vitro lymphocyte proliferation upon stimulation with mitogens is seen in less than 20% of patients. Humoral immunity may also be affected, with deficiencies in immunoglobulin A (IgA) and immunoglobulin G (IgG) subclasses being most common.15,16 Defective immunity plays an important role in the morbidity and mortality of these patients. Mäkitie et al17-19 reported a 21-fold increased risk of mortality in CHH patients, mostly as a result of infections, especially in younger patients. There is also an increased risk of malignancies, particularly non-Hodgkin lymphoma and basal cell carcinoma. The prognosis of these patients after development of malignancies is poor.18 Long-term impairment of regulatory and cytotoxic T-lymphocyte immunity may explain the prevalence of Epstein-Barr virus (EBV)–associated malignant lymphomas and immune dysregulation causing autoimmune diseases in CHH patients.20,21 Kavadas et al22 showed that RMRP mutations are responsible for a variable spectrum of immunodeficiencies and should be considered even in patients without skeletal dysplasias.
Hematopoietic stem cell transplantation (HSCT) has profoundly changed the natural history of severe T-lymphocyte deficiencies by curing many patients with severe combined immunodeficiency (SCID).23,24 Patients with more moderate T-lymphocyte dysfunction (eg, CD40L deficiency) or with phagocyte disorders (eg, chronic granulomatous disease) were also shown to have excellent outcome rates after HSCT, whereas only rarely did they reach the age of 30 with best conventional treatment.25-27 Hence, there is great hope that HSCT-associated restoration of normal T-lymphocyte and natural killer (NK)–cell immunity in CHH patients may also prevent lymphoid malignancy, comparable with patients with Wiskott-Aldrich syndrome, X-linked lymphoproliferative disease, Nijmegen breakage syndrome, Immunodeficiency-Centromeric instability–Facial anomalies syndrome or Dyskeratosis congenita after successful HSCT.28-34 In addition, HSCT should effectively prevent immune dysregulation and autoimmunity as shown previously in other immunodeficiencies and rheumatic diseases.35
Previous single case reports have demonstrated that allogeneic HSCT is able to correct the combined immunodeficiency in CHH patients; however, long-term outcome after HSCT are lacking and particularly the outcome of autoimmune disease in larger cohorts of CHH patients remains unknown.36-38 In this European collaborative survey, we describe the largest cohort of CHH patients (n = 16) who underwent allogeneic HSCT with a minimum follow-up of 1 year, and analyze the characteristics, complications, and the long-term outcome of this procedure.
Methods
Data collection
All members of the Inborn Errors Working Party of the European Bone Marrow Transplantation (EBMT) group were contacted, and data were retrospectively collected from 9 European institutions with 16 CHH patients treated with allogeneic HSCT between 1991 and 2006. All participating institutions approved this retrospective study. Indication for HSCT, age, donor type, conditioning regimen, immune-reconstitution, post-HSCT complications (including graft-versus-host disease [GVHD] and growth, orthopedic, and lung problems), and outcomes were reviewed from medical records. Data analysis was performed in October 2009.
Lymphocyte subsets analysis
Lymphocyte markers were measured as previously described. Briefly, blood was incubated with CD3, CD4, CD8, CD19, and CD56 monoclonal antibodies (Beckman Coulter), erythrocytes were lysed, and cells suspended in a solution with phosphate-buffered saline/bovine serum albumin and sodium azide. Results were analyzed with the Cytomics FC 500 (Beckman Coulter).
Lymphocyte function analysis
T-lymphocyte proliferation was evaluated in vitro after exposure to phytohemagglutinin, Concanavalin A, and pokeweed. After 72 hours, T-lymphocyte proliferative activity was determined by measuring the incorporation of tritiated thymidine into newly synthesized DNA.
Statistical analysis
The Wilcoxon signed ranks test (SPSS 17) was used to compare the number of lymphocyte subpopulations before and after HSCT. A P value of .05 or less was considered to be significant.
Clinical and immunologic characteristics
Patient characteristics are shown in Table 1. Diagnosis of CHH was established on classical clinical features in all but 2 patients. In these 2 patients, the diagnosis was done on genetic basis because they were very young and had no characteristics of skeletal dysplasia. Mutations in RMRP were found in 12 patients of whom 9 were compound heterozygotes.
Clinical and immunologic characteristics of CHH patients
| Patient no. . | Sex . | Mutation . | Clinical characteristics . | Presence before HSCT . | Age at transplantation, y . | Donor . | Conditioning . | Graft stability at last control . | Transplant-related complication . | Outcome . | Follow-up, y . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autoimmunity . | BM failure . | Organ sequlae . | Up to 1 y after HSCT . | Beyond 1 y after HSCT . | ||||||||||
| 1 | F | 12-25 dup/+post(STOP)T | Omenn syndrome | No | No | No | < 1 (8 mo) | MSD | Busulfan/cyclophosphamide | 90% donor on T cells30% donor on B cells70% on myeloid cells | None | None | Alive and well | 11 |
| 2 | F | A214T/G146A | Omenn syndrome | No | No | No | < 1 (8 mo) | Haploidentical father | Busulfan/cyclophosphamide | 100% donor on lymphocytes100% recipient on myeloid cells | None | Severe autoimmune thrombopenia leading to cerebral hemorrhage; CMV primoinfection with lung disease hemorrhage | Died 4 y after HSCT due to autoimmunity | 4 |
| 3 | F | 40G>A/+63C>T | Chronic intractable diarrhea with failure to thrive | Autoimmune enteropathy | Yes, transient | No | 1.9 | MUD | Busulfan/cyclophosphamide | Donor chimerism 100% (whole blood) | EBV-lymphoproliferative disorder | None | Alive and well | 8.5 |
| 4 | M | n.a. | Adenovirus hepatitis; failure to thrive | No | Yes | Yes, liver function disorder due to adenovirus hepatitis | 2.3 | Haploidentical mother | Busulfan/fludarabine | n.a. | Engraftment failure; Klebsiella sepsis; generalized adenovirus infection | n.a. | Died 6 mo after HSCT due to adenovirus infection | n.a |
| 5 | F | g-25_11trip/g.96_97dup | Multiple bacterial pneumonias; varicella pneumonitis; growth hormone deficiency | Yes, autoimmune hemolytic anemia | No | Yes, lung bronchiectasis | 2.5 | MSD | Busulfan/cyclophosphamide | 100% donor (whole blood) | None | None(growth hormone deficiency treated? growth hormone insufficiency substitution) | Alive and well | 16 |
| 6 | M | n.a. | Recurrent lung infections | No | No | Yes, lung bronchiectasis | 2.5 | Haploidentical father | Busulfan/fludarabine/Thiotepa | n.a. | Engraftment failure; generalized adenovirus infection | n.a. | Died 7 mo after HSCT due to adenovirus infection | n.a. |
| 7 | M | 0-4 dup/−13-22tripl | Recurrent fungal infections; chronic diarrhea with failure to thrive | No | No | No | 2.5 | MUD | Busulfan/cyclophosphamide | 100% donor chimerism (whole blood) | Acute GVHD grade II | Acute nephritis: 6 y post-HSCT | Alive and well | 7 |
| 8 | F | 35C>7/70A>G | Pure red cell aplasia | Yes, erythrodermia | Yes | No | 2.5 | MUD | Busulfan/cyclophosphamide | 100% donor (whole blood) | Mild veno-occlusive disease; mild acute GVHD | Pneumococcus sepsis | Died 29 mo after HSCT due to pneumococcus sepsis | 2.5 |
| 9 | M | Homozygous 70 A>G | Persistent EBV viremia | No | No | No | 2.6 | MSD | Busulfan/cyclophosphamide | 100% donor (blood) | None | None | Alive and well | 2.5 |
| 10 | F | insT195/C63T | Several pneumonias and chronic diarrhea; development of aplastic anemia | No | Yes, aplastic anemia; no virus was found as etiology | No | 3 | MSD | Busulfan/cyclophosphamide | 100% donor (whole blood: mononuclear cells, granulocytes) | None | None | Alive and well | 12 |
| 11 | F | 154 G>C | Persistent infection with EBV, herpes simplex virus, and CMV | No | No | Bilateral interstitial pneumonitis and bronchiectasis on high-resolution computed tomography | 3.5 | mMUD | Campath/treosulfan/cyclophosphamide | 100% donor (whole blood) | Pulmonary hemorrhage and multiorgan failure leading to death at day +32 | n.a. | n.a. | |
| 12 | F | Homozygous 70 A>G | Persistent EBV viremia; Adenovirus enteritis; HHV-6 viremia infection of the gut with small round structured virus | No | Yes, EBV-related red cell aplasia | No | 4.5 | MUD | Busulfan/cyclophosphamide | Donor chimerism94% on T cells, 90% on B cells, 76% on myeloid cells | Pneumonitis; HHV-6 infection | None | Alive and well | 7 |
| 13 | M | g.-20_4dup/g.4C>T | Chronic lung and ear infections; intestinal malabsorption with severe failure to thrive; growth hormone deficiency | Yes, autoimmune hemolytic anemia, autoimmune hypothyroidism, and autoimmune enteropathy | No | Yes, lung bronchiectasis | 5.9 | MUD | Busulfan/fludarabine | Donor chimerism 94% on mononuclear cells, 80% on neutrophils | Cutaneous candida infection; BK viremia | Persisting growth hormone deficiency (substituted) | Alive and well | 5 |
| 14 | F | n.a. | Severe varicella infection; failure to thrive; EBV-related large B cell lymphoma with central nervous system compromise | Yes, severe eczema | Yes, transient | No | 11 | MUD | Fludarabine/melphalan | n.a. | Gram-positive sepsis; lung aspergillosis; hemorrhagic cystitis due to BK virus | None | Alive and well | 4 |
| 15 | M | 4C>T/13db dup | Chronic diarrhea; persistent EBV infection; chronic human papillomavirus infection; chronic granulomatous ulcerative skin lesions | No | No | Yes, chronic interstitial pneumonitis and bronchiectasis with oxygen needed at night | 16.8 | MUD | Fludarabine/melphalan | n.a. | Mild cutaneous GVHD | None | Alive and well | 1.6 |
| 16 | F | n.a. | Severe varicella infection; EBV-related non-Hodgkin lymphoma; chronic granulomatous ulcerative skin lesions | No | Yes, CMV-related infection | Yes, lung bronchiectasis secondary to varicella pneumonitis and primary lung lymphoma | 19 | MSD | Busulfan/cyclophosphamide | 100% donor (whole blood) | Lung aspergillosis and candidiasis; CMV gastrointestinal disease; sinocerebral mucormycosis | None | Died 10 mo after HSCT due to cerebral mucormycosis | n.a. |
| Patient no. . | Sex . | Mutation . | Clinical characteristics . | Presence before HSCT . | Age at transplantation, y . | Donor . | Conditioning . | Graft stability at last control . | Transplant-related complication . | Outcome . | Follow-up, y . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Autoimmunity . | BM failure . | Organ sequlae . | Up to 1 y after HSCT . | Beyond 1 y after HSCT . | ||||||||||
| 1 | F | 12-25 dup/+post(STOP)T | Omenn syndrome | No | No | No | < 1 (8 mo) | MSD | Busulfan/cyclophosphamide | 90% donor on T cells30% donor on B cells70% on myeloid cells | None | None | Alive and well | 11 |
| 2 | F | A214T/G146A | Omenn syndrome | No | No | No | < 1 (8 mo) | Haploidentical father | Busulfan/cyclophosphamide | 100% donor on lymphocytes100% recipient on myeloid cells | None | Severe autoimmune thrombopenia leading to cerebral hemorrhage; CMV primoinfection with lung disease hemorrhage | Died 4 y after HSCT due to autoimmunity | 4 |
| 3 | F | 40G>A/+63C>T | Chronic intractable diarrhea with failure to thrive | Autoimmune enteropathy | Yes, transient | No | 1.9 | MUD | Busulfan/cyclophosphamide | Donor chimerism 100% (whole blood) | EBV-lymphoproliferative disorder | None | Alive and well | 8.5 |
| 4 | M | n.a. | Adenovirus hepatitis; failure to thrive | No | Yes | Yes, liver function disorder due to adenovirus hepatitis | 2.3 | Haploidentical mother | Busulfan/fludarabine | n.a. | Engraftment failure; Klebsiella sepsis; generalized adenovirus infection | n.a. | Died 6 mo after HSCT due to adenovirus infection | n.a |
| 5 | F | g-25_11trip/g.96_97dup | Multiple bacterial pneumonias; varicella pneumonitis; growth hormone deficiency | Yes, autoimmune hemolytic anemia | No | Yes, lung bronchiectasis | 2.5 | MSD | Busulfan/cyclophosphamide | 100% donor (whole blood) | None | None(growth hormone deficiency treated? growth hormone insufficiency substitution) | Alive and well | 16 |
| 6 | M | n.a. | Recurrent lung infections | No | No | Yes, lung bronchiectasis | 2.5 | Haploidentical father | Busulfan/fludarabine/Thiotepa | n.a. | Engraftment failure; generalized adenovirus infection | n.a. | Died 7 mo after HSCT due to adenovirus infection | n.a. |
| 7 | M | 0-4 dup/−13-22tripl | Recurrent fungal infections; chronic diarrhea with failure to thrive | No | No | No | 2.5 | MUD | Busulfan/cyclophosphamide | 100% donor chimerism (whole blood) | Acute GVHD grade II | Acute nephritis: 6 y post-HSCT | Alive and well | 7 |
| 8 | F | 35C>7/70A>G | Pure red cell aplasia | Yes, erythrodermia | Yes | No | 2.5 | MUD | Busulfan/cyclophosphamide | 100% donor (whole blood) | Mild veno-occlusive disease; mild acute GVHD | Pneumococcus sepsis | Died 29 mo after HSCT due to pneumococcus sepsis | 2.5 |
| 9 | M | Homozygous 70 A>G | Persistent EBV viremia | No | No | No | 2.6 | MSD | Busulfan/cyclophosphamide | 100% donor (blood) | None | None | Alive and well | 2.5 |
| 10 | F | insT195/C63T | Several pneumonias and chronic diarrhea; development of aplastic anemia | No | Yes, aplastic anemia; no virus was found as etiology | No | 3 | MSD | Busulfan/cyclophosphamide | 100% donor (whole blood: mononuclear cells, granulocytes) | None | None | Alive and well | 12 |
| 11 | F | 154 G>C | Persistent infection with EBV, herpes simplex virus, and CMV | No | No | Bilateral interstitial pneumonitis and bronchiectasis on high-resolution computed tomography | 3.5 | mMUD | Campath/treosulfan/cyclophosphamide | 100% donor (whole blood) | Pulmonary hemorrhage and multiorgan failure leading to death at day +32 | n.a. | n.a. | |
| 12 | F | Homozygous 70 A>G | Persistent EBV viremia; Adenovirus enteritis; HHV-6 viremia infection of the gut with small round structured virus | No | Yes, EBV-related red cell aplasia | No | 4.5 | MUD | Busulfan/cyclophosphamide | Donor chimerism94% on T cells, 90% on B cells, 76% on myeloid cells | Pneumonitis; HHV-6 infection | None | Alive and well | 7 |
| 13 | M | g.-20_4dup/g.4C>T | Chronic lung and ear infections; intestinal malabsorption with severe failure to thrive; growth hormone deficiency | Yes, autoimmune hemolytic anemia, autoimmune hypothyroidism, and autoimmune enteropathy | No | Yes, lung bronchiectasis | 5.9 | MUD | Busulfan/fludarabine | Donor chimerism 94% on mononuclear cells, 80% on neutrophils | Cutaneous candida infection; BK viremia | Persisting growth hormone deficiency (substituted) | Alive and well | 5 |
| 14 | F | n.a. | Severe varicella infection; failure to thrive; EBV-related large B cell lymphoma with central nervous system compromise | Yes, severe eczema | Yes, transient | No | 11 | MUD | Fludarabine/melphalan | n.a. | Gram-positive sepsis; lung aspergillosis; hemorrhagic cystitis due to BK virus | None | Alive and well | 4 |
| 15 | M | 4C>T/13db dup | Chronic diarrhea; persistent EBV infection; chronic human papillomavirus infection; chronic granulomatous ulcerative skin lesions | No | No | Yes, chronic interstitial pneumonitis and bronchiectasis with oxygen needed at night | 16.8 | MUD | Fludarabine/melphalan | n.a. | Mild cutaneous GVHD | None | Alive and well | 1.6 |
| 16 | F | n.a. | Severe varicella infection; EBV-related non-Hodgkin lymphoma; chronic granulomatous ulcerative skin lesions | No | Yes, CMV-related infection | Yes, lung bronchiectasis secondary to varicella pneumonitis and primary lung lymphoma | 19 | MSD | Busulfan/cyclophosphamide | 100% donor (whole blood) | Lung aspergillosis and candidiasis; CMV gastrointestinal disease; sinocerebral mucormycosis | None | Died 10 mo after HSCT due to cerebral mucormycosis | n.a. |
CHH was established on classical clinical features in all but 2 study patients.
AIHA indicates autoimmune hemolytic anemia; HHV-6, human herpesvirus type 6; mMUD, mismatched MUD; MSD, matched sibling donor; and n.a., not available.
All patients had combined immunodeficiency with or without autoimmune features before HSCT. Immunodeficiency was the main indication for HSCT. Two patients underwent transplantation after the development of EBV-related non-Hodgkin lymphoma associated with central nervous system compromise (patient 14 [pt 14]) and relapsed non-Hodgkin lymphoma and CMV-induced bone marrow failure (pt 16). Pre-existing autoimmunity and inflammatory skin lesions were a prominent feature in 5 of 16 patients (patient nos. 3, 5, 8, 11, and 12; Table 1). Thirteen patients were transplanted in early childhood (mean age 2.5 years). Three patients underwent transplantation in adolescent age (pt 14, pt 15, and pt 16).
Pretransplantation immunologic abnormalities are summarized in Table 2. A striking abnormality was lymphopenia with impaired T-lymphocyte immunity (median lymphocyte count 1043 cells/μL, range 267-2030). Patient values in comparison with normal median numbers for age are shown in Table 3. T-lymphocyte subsets were more affected than B lymphocytes and NK cells. The median CD3-cell number was 295 cells/μL (range 50-1400). The CD4-cell counts had a median of 189/μL (range 40-960) while the median of CD8+ cells was 95/μL (range 4-568). Mitogen-induced lymphocyte proliferation test values ranged from very low to absent in all but 1 patient (pt 1).
Lymphocyte populations and function before HSCT and number of cells and immunoglobulin levels before transplantation
| . | Pt 1 . | Pt 2 . | Pt 3 . | Pt 4 . | Pt 5 . | Pt 6 . | Pt 7 . | Pt 8 . | Pt 9 . | Pt 10 . | Pt 11 . | Pt 12 . | Pt 13 . | Pt 14 . | Pt 15 . | Pt 16 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | < 1 (8 mo) | < 1 (8 mo) | 1.9 | 2 | 2.3 | 2.5 | 2.5 | 2.5 | 2.6 | 3 | 2.7 | 4.5 | 5.9 | 11 | 16.8 | 19 |
| Lymphocytes/μL | 1778 | 275 | 314 | 600 | 1100 | 1250 | 1105 | 1100 | 1494 | 288 | 1087 | 723 | 312 | 2030 | 1000 | 267 |
| CD3+/μL | 1120 | 290 | 113 | 50 | 300 | 99 | 105 | 655 | 638 | 72 | 886 | 583 | 154 | 1400 | 470 | 215 |
| CD4+/μL | 960 | 210 | 85 | 40 | 100 | 62 | 101 | 545 | 417 | 55 | 336 | 389 | 138 | 832 | 340 | 168 |
| CD8+/μL | 160 | 80 | 28 | 10 | 200 | 37 | 4 | 110 | 221 | 17 | 278 | 194 | 16 | 568 | 130 | 47 |
| C19+/μL | 391 | 90 | 395 | 300 | 600 | 200 | 152 | 220 | 334 | 144 | 0* | 311 | 21 | 466 | 270 | 68 |
| NK cells/μL | 243 | n.a. | 229 | n.a. | 221 | n.a. | 141 | 250 | 212 | 32 | 201 | 914 | 77 | 101 | 230 | 258 |
| IgG | Low | Low | Normal | Normal | Low | Normal | Normal | Normal | Normal | Normal | Under IVIG | Normal | Low | Normal | Normal | Normal |
| IgM | Normal | Low | Normal | Low | Normal | Normal | Normal | Normal | Normal | Normal | Low | Normal | Low | Low | Normal | Normal |
| IgA | Normal | Low | Normal | Low | Low | Normal | Normal | Normal | Low | Normal | Low | Low | Low | Low | Normal | Normal |
| Response to mitogens | n.a. | Poor | Absent | Absent | Poor | Poor | Absent | Poor | Normal | Poor | Poor | Poor | Poor | Poor | Absent | Poor |
| . | Pt 1 . | Pt 2 . | Pt 3 . | Pt 4 . | Pt 5 . | Pt 6 . | Pt 7 . | Pt 8 . | Pt 9 . | Pt 10 . | Pt 11 . | Pt 12 . | Pt 13 . | Pt 14 . | Pt 15 . | Pt 16 . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | < 1 (8 mo) | < 1 (8 mo) | 1.9 | 2 | 2.3 | 2.5 | 2.5 | 2.5 | 2.6 | 3 | 2.7 | 4.5 | 5.9 | 11 | 16.8 | 19 |
| Lymphocytes/μL | 1778 | 275 | 314 | 600 | 1100 | 1250 | 1105 | 1100 | 1494 | 288 | 1087 | 723 | 312 | 2030 | 1000 | 267 |
| CD3+/μL | 1120 | 290 | 113 | 50 | 300 | 99 | 105 | 655 | 638 | 72 | 886 | 583 | 154 | 1400 | 470 | 215 |
| CD4+/μL | 960 | 210 | 85 | 40 | 100 | 62 | 101 | 545 | 417 | 55 | 336 | 389 | 138 | 832 | 340 | 168 |
| CD8+/μL | 160 | 80 | 28 | 10 | 200 | 37 | 4 | 110 | 221 | 17 | 278 | 194 | 16 | 568 | 130 | 47 |
| C19+/μL | 391 | 90 | 395 | 300 | 600 | 200 | 152 | 220 | 334 | 144 | 0* | 311 | 21 | 466 | 270 | 68 |
| NK cells/μL | 243 | n.a. | 229 | n.a. | 221 | n.a. | 141 | 250 | 212 | 32 | 201 | 914 | 77 | 101 | 230 | 258 |
| IgG | Low | Low | Normal | Normal | Low | Normal | Normal | Normal | Normal | Normal | Under IVIG | Normal | Low | Normal | Normal | Normal |
| IgM | Normal | Low | Normal | Low | Normal | Normal | Normal | Normal | Normal | Normal | Low | Normal | Low | Low | Normal | Normal |
| IgA | Normal | Low | Normal | Low | Low | Normal | Normal | Normal | Low | Normal | Low | Low | Low | Low | Normal | Normal |
| Response to mitogens | n.a. | Poor | Absent | Absent | Poor | Poor | Absent | Poor | Normal | Poor | Poor | Poor | Poor | Poor | Absent | Poor |
Pt indicates patient; IVIG, intravenous immunoglobulin; and n.a., not available.
Previous rituximab.
Lymphocyte populations and function in survivors and immunoglobulin levels at last follow-up after HSCT
| . | Pt 1 . | Pt 3 . | Pt 5 . | Pt 7 . | Pt 9 . | Pt 10 . | Pt 12 . | Pt 13 . | Pt 14 . | Pt 15 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 11 | 8 | 17 | 7 | 4 | 15 | 11 | 10 | 14 | 17 |
| Lymphocytes/μL | 1130 | 2520 | 3757 | 2662 | 2758 | 4410 | 1070 | 2600 | 2250 | 2200 |
| CD3+/μL | 654 | 1714 | 2454 | 2336 | 1933 | 2293 | 817 | 1700 | 1552 | 1514 |
| CD4+/μL | 386 | 857 | 1554 | 979 | 1130 | 794 | 551 | 1080 | 900 | 264 |
| CD8+/μL | 268 | 857 | 900 | 1357 | 803 | 1323 | 266 | 620 | 652 | 1250 |
| C19+/μL | 164 | 529 | 500 | 226 | 473 | 573 | 65 | 520 | 495 | 285 |
| NK cells/μL | 175 | n.a. | 300 | 119 | 291 | 1411 | 95 | 180 | 67 | 330 |
| IgG | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal (Low IgG2) | Normal | Normal |
| IgM | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| IgA | Normal | Absent | Normal | Low | Normal | Normal | Absent | Absent | Absent | Normal |
| Response to mitogens | Normal | Normal | Improved | Normal | Normal | Normal | Improved | Improved | Improved | Improved |
| . | Pt 1 . | Pt 3 . | Pt 5 . | Pt 7 . | Pt 9 . | Pt 10 . | Pt 12 . | Pt 13 . | Pt 14 . | Pt 15 . |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 11 | 8 | 17 | 7 | 4 | 15 | 11 | 10 | 14 | 17 |
| Lymphocytes/μL | 1130 | 2520 | 3757 | 2662 | 2758 | 4410 | 1070 | 2600 | 2250 | 2200 |
| CD3+/μL | 654 | 1714 | 2454 | 2336 | 1933 | 2293 | 817 | 1700 | 1552 | 1514 |
| CD4+/μL | 386 | 857 | 1554 | 979 | 1130 | 794 | 551 | 1080 | 900 | 264 |
| CD8+/μL | 268 | 857 | 900 | 1357 | 803 | 1323 | 266 | 620 | 652 | 1250 |
| C19+/μL | 164 | 529 | 500 | 226 | 473 | 573 | 65 | 520 | 495 | 285 |
| NK cells/μL | 175 | n.a. | 300 | 119 | 291 | 1411 | 95 | 180 | 67 | 330 |
| IgG | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal (Low IgG2) | Normal | Normal |
| IgM | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| IgA | Normal | Absent | Normal | Low | Normal | Normal | Absent | Absent | Absent | Normal |
| Response to mitogens | Normal | Normal | Improved | Normal | Normal | Normal | Improved | Improved | Improved | Improved |
Pt indicates patient; and n.a., not available.
The B-lymphocyte count was low in 2 patients (pt 2 and pt 11); the data of 1 patient (pt 11) are not available because he received Rituximab before the evaluation. Serum IgG, IgM, and IgA levels were rather variable. Levels were normal in 7 patients, and in 2, all fractions were reduced. A combination of IgM and IgA low levels was seen in 2 patients. Isolated IgG deficiency was present in 1 patient and combined IgG and IgA deficiency was observed in 1 patient. Isolated IgA deficiency was detected in 2 patients. Pt 11 was under immunoglobulin substitution after Rituximab administration.
Transplantation procedures
All patients were nursed in high-efficiency particulate absorbing filtered rooms or in an isolator and received prophylaxis with intravenous immunoglobulins and trimethoprim/sulfamethoxazole against Pneumocystis jirovecii infection.
Thirteen patients received myeloablative conditioning with busulfan (16-20 mg/kg in 4 divided doses) and cyclophosphamide (120-200 mg/kg). Two patients received fludarabine-based reduced-intensity conditioning and 1 patient received treosulfan-based conditioning. Cyclosporine A and a short course of methotrexate were most frequently used for GVHD prophylaxis. Anti–T-lymphocyte globulin or alemtuzumab was used in matched unrelated donors (MUDs) and 2 haploidentical HSCTs, and 1 patient received OKT3 (Table 1).
Informed consent was given before HSCT in accordance with the Declaration of Helsinki.
Results
Five patients underwent HSCT with HLA-identical siblings, 8 had MUDs, and 3 had haploidentical donors. A total of 13 of 14 patients who received myeloablative conditioning, and 2 of 3 who received reduced-intensity conditioning engrafted. Both types of conditioning regimen were well tolerated. No severe hepatic veno-occlusive disease occurred. There was no grade 3/4 GVHD.
A total of 6 of 16 patients (37%) died, of whom 3 patients had received extensive lymphocyte-depleted grafts from haploidentical donors, 2 patients with matched unrelated, and 1 patient after matched sibling transplantation. A total of 4 patients died during the first year after HSCT, one due to disseminated adenovirus infection in myeloid aplasia, the other 3 patients due to infectious complications despite full donor chimerism The survival rate for the matched sibling and unrelated donor transplantation was 10/13 (80%; Table 1).
There were 2 late deaths observed, both with incomplete immune reconstitution; 1 patient with pneumococcal sepsis 2.5 years post-HSCT, and 1 patient 4 years post-HSCT with cytomegalovirus (CMV) infection and severe autoimmune thrombocytopenia leading to lethal cerebral hemorrhage.
During and after HSCT, viral and fungal infectious were major problems and occurred in 8 patients (50%). Two patients died from disseminated adenovirus disease. Two patients had Polyoma BK viral infections, one with blood viremia and one with hemorrhagic cystitis only. One patient each had CMV disease with pneumonitis, EBV-associated lymphoproliferative disease, and human herpes virus type 6 viremia. Invasive fungal infections were seen in 2 patients, one with lung aspergillosis and one with a multifungal infection of the lung, with Candida and Aspergillus species followed by untreatable sinocerebral mucormycosis, which was lethal at 10 months after transplantation despite full donor chimerism.
Characteristics of patients with long-term follow-up
The 10 survivors are well and have normal immunity The median follow-up in the 10 long-term survivors was 7 years (range 1.6-16 years).
Immunologic characteristics after HSCT
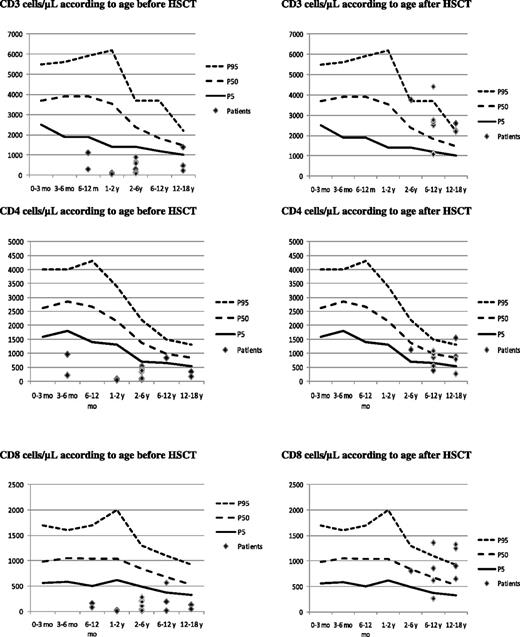
The major impact of the HSCT was seen in the improvement of the number and function of the lymphocytes (Table 3; Figure 1). The median CD3-lymphocyte count at last follow-up was 1552/μL (P = .006, Wilcoxon signed rank test). The median CD4-lymphocyte count was 857/μL (P = .01, Wilcoxon signed rank test), and the median CD8-lymphocyte count was 652/μL (P = .003, Wilcoxon signed rank test).
Lymphocyte counts by patient related to age before and after HSCT. Top left panel shows CD3 cells per μL before HSCT, and top right after HSCT. Middle left panel shows CD4 per μL before HSCT, and middle right after HSCT. Bottom left panel shows CD8 per μL before HSCT, and bottom right after HSCT.
Lymphocyte counts by patient related to age before and after HSCT. Top left panel shows CD3 cells per μL before HSCT, and top right after HSCT. Middle left panel shows CD4 per μL before HSCT, and middle right after HSCT. Bottom left panel shows CD8 per μL before HSCT, and bottom right after HSCT.
The lymphocyte proliferation to antigen stimulation normalized or improved in all patients. The response to specific antigens was normal in all long-term survivors.
The improvement in the B-lymphocyte and NK-cell counts was not statistically significant. Four patients showed a remaining isolated IgA deficiency (pt 2, pt 12, and pt 14), and 1 patient with no successful donor immune reconstitution needed continued intravenous immunoglobulin substitution until death 4 years post-HSCT.
Characteristics of the donor chimerism
Donor chimerism was assessed in 12 patients, and was measured in whole blood in some centers and as lineage-specific chimerism in other centers. In patients in whom whole blood chimerism was performed, donor chimerism was 100%; in those patients with cell line–specific chimerism, CD3 chimerism was 90% donor or more and myeloid cell-lineage donor chimerism was 70% or more (Table 1).
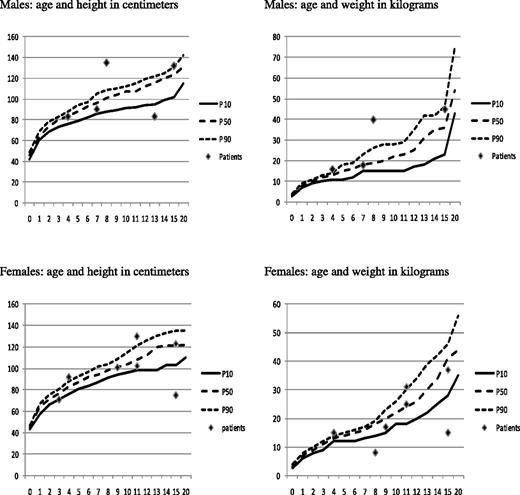
A total of 7 patients had pre-existing moderate bronchiectasis before HSCT. After HSCT, lung function stabilized or improved and pre-existing bronchiectasis did not progress. HSCT had no major impact on the growth and development of these patients (Figure 2). All but 2 patients, who were below fifth percentile before HSCT, reached the expected CHH-length percentile for age (see also growth curves39 ). One patient (pt 13) had persisting growth hormone deficiency after HSCT, and growth hormone was recently substituted. Patients with CHH and recurrent lung infection are known to have less growth potential than other CHH patients. It is remarkable, therefore, that the majority of our patients achieved catch-up growth for the disease after HSCT, probably because immunologic improvement led to a reduction in the frequency of lung infection (growth curves). The intellectual development is normal in all patients. None have developed malignancy or de novo autoimmune disease to date.
Growth curves of patients with cartilage hair hypoplasia at last follow-up after HSCT. Growth curves based upon the study of Mäkitie et al.39 Top left panel shows height corresponding with age in males, and top right shows weight corresponding with age. Bottom left panel shows height corresponding with the age in females, and the right panel shows weight corresponding with age.
Growth curves of patients with cartilage hair hypoplasia at last follow-up after HSCT. Growth curves based upon the study of Mäkitie et al.39 Top left panel shows height corresponding with age in males, and top right shows weight corresponding with age. Bottom left panel shows height corresponding with the age in females, and the right panel shows weight corresponding with age.
Discussion
The first report of a patient with CHH who underwent HSCT showed a clear benefit in terms of restoring impaired immunity, but no improvement of the associated skeletal problems.36,37 Guggenheim et al38 also demonstrated that HSCT provides prolonged engraftment and stable immune reconstitution in 3 patients with CHH and combined immunodeficiency.
We describe the largest so far reported cohort of patients with CHH who underwent HSCT. The patients were characterized by T-lymphocyte immunodeficiency of various degrees, contributing to infectious complications, autoimmune disorders, and lymphoid malignancies. In addition, 2 patients presented with clinical characteristics of Omenn syndrome.
The overall survival rate was 10/16 (63%) and 10/13 (80%) for the patients receiving matched sibling transplants or unrelated donor transplants. These results are in keeping with the European results for other T-cell immune deficiency, and highlight the poor results from haploidentical HSCT, but encouraging results when well-matched donors are used.
Conditioning regimens with busulfan and cyclophosphamide were effective and well tolerated. Fludarabine-based reduced-intensity conditioning regimens were used more recently, in particularly in patients who already have significant organ damage with higher risk for transplant-related mortality. The limited number of patients treated by reduced-intensity conditioning in this series does not permit a draw to conclusions on the efficacy of this approach in CHH.
Early work of Pierce and Polmar40,41 clearly showed that there was an intrinsic defect in the proliferative capacity of RMRP-deficient lymphocytes, which was confirmed by work of Kooijman et al.42 Exactly how the RMRP mutations affect the cell-cycle is not fully understood, but data strongly suggest that there is a partial cell defect in the transition from G0 to the G1 phase and/or defects in the progression through the G1 phase. This could explain at least in part the severe lymphocytopenia in some patients with CHH and the observed low lymphocyte proliferation to specific mitogens in vitro. In addition, the recent demonstration that RMRP forms a complex with TERT provides strong evidence to a role of RMRP in prevention of cellular senescence and maintenance of telomere homeostasis.6
Reduced thymic output of patients with CHH and combined immunodeficiency, measured by T-lymphocyte receptor excision circles, has been demonstrated.22 Furthermore, it has been recently shown that thymic abnormalities in these patients also include reduced expression of aire (a transcription factor involved in clonal deletion of self-reactive T cells) and poor generation of thymic natural regulatory T cells.43 These abnormalities are likely involved in the autoimmune manifestations of CHH. Autoimmunity is well described in other immunodeficiencies with impaired thymic output including partial DiGeorge syndrome and Omenn syndrome.44 Autoimmunity, mostly hematologic, has been described in patients with CHH and was common in our cohort. It is possible that the frequency of autoimmunity has been underestimated in patients with CHH and should be actively sought for.44-46 The immune dysregulation was successfully corrected in all but 1 patient who had impaired immune reconstitution (pt 13) in whom severe autoimmune thrombocytopenia led to cerebral hemorrhage and death. Inflammatory skin lesions were also normalized post-HSCT.
Bone marrow hypoplasia, often transient, has been described in patients with CHH. In our group of patients, 5 of 16 patients developed various degrees of bone marrow failure. The etiology of this complication could be related to RMRP-associated cell-cycle regulation, but viral infections should also be a factor. In all such cases, this HSCT successfully restored bone marrow function and cellularity.
Transplantation-related mortality in our cohort was mostly a result of infectious complications. Two late deaths were seen, with 1 death at 2.5 years post-HSCT because of pneumococcus sepsis and 1 death at 4 years from severe autoimmune thrombocytopenia associated with CMV lung disease. Long-term follow-up with careful assessment of T- and B-lymphocyte function is therefore necessary.
After successful HSCT, there was a significant increase of the T-lymphocyte numbers, with only minor changes of B lymphocyte and NK–cell numbers. Donor chimerism remains stable and the lymphocyte function was improved in all patients. All survivors attend normal schools and have a good quality of life.
Patients with RMRP mutations showed a striking variability in clinical manifestation even within sibships, with no genotype/phenotype correlation,17 contributing to the challenge in selecting those patients who will benefit from HSCT, while not selecting those who do not require the procedure. With our current understanding, not all CHH patients should be considered for HSCT but only those with chronic or recurrent infection in association with autoimmunity or bone marrow hypoplasia if a well-matched donor is available. Patients with CHH should have careful assessment of lymphocyte numbers and function, but those with normal results should be considered for HSCT in the event of evolving infections, autoimmunity and/or blood cytopenias, before development of significant organ damage or malignancy. It is questionable whether haploidentical HSCT should be considered in this setting. In the case of lacking compatible unrelated donor, unrelated cord blood transplantations should be investigated as a true alternative for haploidentical transplantations. This could have a major impact on the survival and quality of life of young CHH patients. In the meantime, cohort studies with genotype-phenotype correlations are urgently needed to address the question that patients will benefit most from the procedure.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.B. designed the research, contributed and acquired the clinical and immunologic data, performed the statistical analysis, and wrote the manuscript. A.R.G., M.A.S., and T.G. designed the research, contributed clinical and immunologic data, contributed statistical analysis, and corrected the manuscript. E.V., G.L., P.V., Q.W., W.F., N.M.W., F.S., A.J.C., A.F., M.C.-C., R.G.M.B., L.D.N., E.M., and B.N. contributed clinical and immunologic data and correction on the manuscript.
On behalf of the Inborn Error Working Party of the European Bone Marrow Transplantation (EBMT) group. For a complete list of participants from the Inborn Error Working Party of the EBMT group, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Victoria Bordon Cueto de Braem, Pediatric Hematology, Oncology, and Stem Cell Transplantation, Kliniek voor Kinderziekten C. Hooft, de Pintelaan 185, 9000 Ghent, Belgium; e-mail: victoria.bordon@uzgent.be. (Please address publication to corresponding author using the name as listed here.)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal