Abstract
Regulatory T cells (Tregs) suppress graft-versus-host disease (GVHD) while preserving a beneficial graft-versus-leukemia (GVL) effect. Thus, their use in allogeneic stem cell transplantation (SCT) provides a promising strategy to treat GVHD. However, 3 obstacles prevent their routine use in human clinical trials: (1) low circulating number of Tregs in peripheral blood, (2) loss of suppressor function after in vitro expansion, and (3) lack of Treg-specific surface markers necessary for efficient purification. FOXP3 is exclusively expressed in Tregs and forced expression in CD4+CD25− T cells can convert these non-Tregs into Tregs with functional suppressor function. Here, we show that the FDA-approved hypomethylating agents, decitabine (Dec) and azacitidine (AzaC), induce FOXP3 expression in CD4+CD25− T cells both in vitro and in vivo. Their suppressor function is dependent on direct contact, partially dependent on perforin 1 (Prf1), but independent of granzyme B (GzmB), and surprisingly, Foxp3. Independence of Foxp3 suggests that genes responsible for the suppressor function are also regulated by DNA methylation. We have identified 48 candidate genes for future studies. Finally, AzaC treatment of mice that received a transplant of major histocompatibility complex mismatched allogeneic bone marrow and T cells mitigates GVHD while preserving GVL by peripheral conversion of alloreactive effector T cells into FOXP3+ Tregs and epigenetic modulation of genes downstream of Foxp3 required for the suppressor function of Tregs.
Introduction
Allogeneic stem cell transplantation (SCT) represents the most effective treatment for patients with marrow failure states and other hematologic malignancies such as acute and chronic leukemias. One of the major complications of allogeneic SCT is graft-versus-host disease (GVHD), caused by donor T cells reacting against host antigens.1 This acute inflammatory reaction can be mild, moderate, or life-threatening especially in recipients of unrelated or human leukocyte antigen–mismatched stem cell products.2 However, these same alloreactive donor T cells provide a beneficial graft-versus-leukemia (GVL) effect, reducing the risk of leukemia relapse.3,4 Therefore, the current clinical goal in treatment of GVHD is to preferentially suppress GVHD while maintaining GVL.
Regulatory T cells (Tregs) are known to contribute to the maintenance of self-tolerance by regulating inflammatory responses and to suppression of autoimmunity and GVHD in mouse models.5-9 The major population of Tregs is naturally occurring Tregs or nTregs. They are generated in the thymus and defined by CD4+CD25+FOXP3+.5-8 Small number of Tregs can also be generated in the periphery from naive CD4+CD25− T cells by T cell–receptor stimulation along with retinoic acid, TGF-β, and IL-10.10,11 Because Tregs can also mitigate GVHD by suppressing alloreactive donor T cells without sacrificing GVL in animal models, their use in the allogeneic transplantation setting provides a promising strategy to treat or mitigate GVHD.9 However, circulating numbers of Tregs in peripheral blood are limited (5%–10% of CD4+ T cells), and despite significant improvements in methodologies for in vitro purification of Tregs, the current protocols for in vitro Treg expansion are inefficient, costly, and time-consuming.12-15 Furthermore, the lack of Treg-specific cell surface markers makes it impossible to purify Tregs expanded in vitro, and expanded Tregs often fail to maintain their suppressor function,13,16 possibly due to the loss of expression of FOXP3 and/or chemokine receptors, such as CXCR3,17 CCR6,18 and CCR819 that facilitate trafficking of Tregs to sites of inflammation.
FOXP3 is a forkhead box transcription factor exclusively expressed in nTregs.5-8 Its mutations lead to autoimmune diseases due to the loss of functional nTregs and forced expression of FOXP3 in CD4+CD25− T cells induces regulatory properties.5,7,8,20-22 These data suggest that Foxp3 is necessary and sufficient for functional nTregs. Recent reports demonstrated that the Foxp3 locus in both humans and mice is unmethylated in Tregs while heavily methylated and silenced in CD4+CD25− T cells.23-25
Dec and AzaC, analogues of 2′-deoxycytidine and cytidine, respectively, are hypomethylating agents that the FDA approved for the treatment of myelodysplastic syndromes. Dec can incorporate into replicating DNA, while AzaC incorporates primarily into RNA with some integration into DNA after 5-aza-ribonucleotides are converted into 5-aza-deoxyribonucleotides by ribonucleotide reductase.26-29 Once incorporated into DNA, they can trap DNA methyltransferase 1 (DNMT1),30 thereby inhibiting DNA methylation.27
Based on these reports, we hypothesized that Dec and AzaC could be used to induce the expression of FOXP3 in CD4+CD25− T cells via epigenetic modification and convert these non-Tregs into Tregs. In this study, we report that these drugs induce the expression of Foxp3 in activated CD4+CD25− T cells generating functional Tregs with suppressor properties. We further demonstrate that in vivo treatment of mice with AzaC after allogeneic SCT dramatically mitigates GVHD while preserving GVL at least in part by increasing the peripheral conversion of CD4+CD25− alloreactive T effector cells (Teffs) into functionally suppressive FOXP3+ Tregs. In addition, the suppressor function of these AzaC-induced Tregs is independent of Foxp3, suggesting that AzaC modifies the expression of not only Foxp3 but also other genes that are necessary for Treg suppressor function. Thus, our study suggests that epigenetic modulation of events distal to Foxp3 is also a critical mechanism by which in vivo administration of AzaC controls GVHD. Our study provides a solid foundation for a pharmacologic therapy to limit GVHD without sacrificing GVL.
Methods
Mice
Balb/c (H-2Kd, CD45.2+) and C57BL/6 (B6; H-2Kb, CD45.2+) mice were obtained from Taconic Farms. Congenic B6 mice expressing the CD45.1 gene were purchased from The Jackson Laboratory. Animal care and euthanasia were approved by the Washington University School of Medicine Animal Studies Committee. Six- to 12-week-old mice were used except for Foxp3 KO experiments in which 3-week-old Foxp3 KO (B6 background) mice5 and littermate control mice were used.
Cell culture
Human peripheral blood mononuclear cells (PBMCs) were harvested by ficoll gradient centrifugation. CD4+CD25− T cells and CD4+CD25+ T cells were isolated from the human PBMCs using Miltenyi microbeads and AutoMACS (Miltenyi Biotec).31 The isolated CD4+CD25− T cells were activated for 2 to 3 days in the presence of anti-CD3/CD28 beads (bead:cell = 1:1; Invitrogen) and Stemline T-cell expansion medium (Sigma-Aldrich) supplemented with L-glutamine (4mM), penicillin (100 U/mL), streptomycin (100 μg/mL), and human recombinant IL-2 (hIL-2; 50 U/mL). The activated T cells were incubated in the presence of Dec (0.1-10μM; Sigma-Aldrich) or phosphate-buffered saline (PBS) for an additional 1 to 4 days. Mouse CD4+CD25−T cells and CD4+CD25+ T cells were isolated from mouse spleens using Miltenyi microbeads and AutoMACS. The isolated CD4+CD25− T cells were activated for 2 days (2 or 4 days for Foxp3 KO and littermate controls) in the presence of beads (bead:cell = 1:1) and Dulbecco modified Eagle medium supplemented with 10% FCS, L-glutamine (2mM), penicillin (100 U/mL), streptomycin (100 μg/mL), MEM nonessential amino acids (1×), sodium pyruvate (1mM), HEPES buffer (20mM), 2-mercaptoethanol (50μM) and hIL-2 (10 U/mL), called Xcyte media. The activated T cells were incubated in the presence of Dec (0.1-10μM), AzaC (0.5-4μM), or PBS for an additional 2 days.
Real-time RT-PCR
Human CD4+CD25− T cells that were activated with beads for 3 days (defined as day 0) and incubated in the presence of Dec or PBS for additional 1 to 4 days (day 1 through day 4) as described in the previous paragraph. Total RNA was isolated from these cells every day (day 0 through day 4) using the RNeasy Plus Mini Kit (QIAGEN). The 7300 Real Time PCR system (Applied Biosystems), QuantiTect Primer Assays primers (GAPDH as an internal control), and QuantiTect SYBR Green RT-PCR kits (QIAGEN) were used.
RNA profiling analysis
Total RNA was isolated from the following cells (all B6, CD45.2+) using TRIzol Reagent (Invitrogen): CD4+CD25+ naive Tregs, CD4+CD25+ Tregs that were incubated in Xcyte media (100 U/mL hIL-2) for 4 days in the presence of the beads (bead:cell = 1:1), PBS-treated T cells (pbsTs), and Dec-induced Tregs (dcTs). Target preparation, gene chip hybridization, and analysis were performed by the Washington University Siteman Cancer Center Gene Chip Facility. Labeled target was made from non-amplified total RNA and was hybridized to Mouse Genome 430 2.0 array (Affymetrix).
Flow cytometric analysis
The following antibodies were used. For human T cells: CD4-APC, CD25-FITC (BD Pharmingen), and FOXP3-PE (clone PCH101; eBioscience), for mouse T cells: CD4-FITC, CD25-PE (BD Pharmingen), Foxp3-PE and Foxp3-PECy5 (clone FJK-16s; eBioscience), and for SCT: H-2Kb-FITC, CD4-PE, CD3-PECy7, B220-APC and CD45.2-biotin/streptavidin APC-Cy7 (BD Pharmingen). All cells were analyzed on a FACScan cytometer (BD Biosciences).
CFSE-based proliferation assays and MLR
CD4+CD25− T cells (B6, CD45.1) were labeled with carboxyfluorescein diacetate, succinimidyl ester (CFSE) at a final concentration of 300nM. The CFSE-labeled cells were incubated in 200 μL of Xcyte media with suppressors, such as CD4+CD25+ nTregs, dcTs, azacTs, or pbsTs, (all B6, CD45.2), and γ-irradiated (20 Gy) splenocytes/antigen presenting cells (APCs; Balb/c, CD45.2) for mixed lymphocyte reaction (MLR) or beads for proliferation assays. 1:1:1 = Teff:bead/APC:suppressor ratio was used unless otherwise indicated. DcTs and azacTs are extensively washed with PBS (2×) before coculture with T effectors, thus T effectors are not exposed to any detectable levels of decitabine or azacitidine.
SCT
SCT was performed as previously described31 with the following modifications. Total-body irradiation (TBI; 900 cGy) using a Mark I cesium irradiator (J. L. Shepherd and Associates) was used to condition recipient mice (Balb/c). B6 mice (CD45.1 or CD45.2) T cell–depleted (TCD) bone marrow (BM; 5 × 106 cells) was used as a stem cell source. To induce GVHD, 5 × 105 B6 conventional CD4+/CD8+ T cells (Tconv; CD45.1) were infused along with donor (B6) TCD BM. To test suppressor function of dcTs, 5 × 105 B6 CD4+CD25+ nTregs (CD45.2; for control), pbsTs or dcTs (CD45.2) generated from CD4+CD25− T cells were injected with TCD BM and Tconv. In some cases, 10 × 106 pbsTs or dcTs (CD45.2) generated from Tconv were given in the place of Tconv and pbsTs or dcTs that were derived from CD4+CD25− T cells. For delayed donor lymphocyte infusions, 2 × 106 or 10 × 106 Tconv were given on day 11 after SCT. For examination of GVL effect, 1 × 104 A20-luc/egfp leukemic cells were given along with TCD BM. Animals losing 20% of their starting body weights were killed.
BLI
GVL effect assessment and bioluminescence imaging (BLI) of animals were done as previously described.31,32 Briefly, mice were injected intraperitoneally with 150 μg/g D-luciferin (Biosynth) in PBS and imaged 10 minutes later. Imaging was done using a charge-coupled device camera (IVIS 100; Caliper Corporation; exposure time, 1-60 seconds; binning, 8; field of view, 15; f/stop 1, open filter) at the Molecular Imaging Center (Washington University). Mice were anesthetized using isoflourane (2.5% vaporized in O2). For analysis, total photon flux (photons per second) was measured from a fixed region of interest over the entire abdomen and thorax using Living Image 2.50 and IgorPro software (Wavemetrics). BLI was performed 1 day before donor T-cell infusion and the first injection of AzaC and 3 days after the last injection of AzaC, then once every week until day 52 after SCT.
Administration of Dec and AzaC
AzaC and Dec were prepared in cold PBS and were injected 4 times subcutaneously or intraperitoneally every other day starting on day 4 after donor lymphocyte infusion (day 15 after SCT). Foxp3 KO mice were also injected 4 times subcutaneously every other day starting at 16 to 26 days of age.
Statistical analysis
The significance of differences in survival of treatment groups was analyzed using the log-rank test. For proliferation assays and MLR, unpaired t test was used. P values less than .05 were considered significant. For RNA profiling analyses, the probes were identified using GA-Mantel method.33 This method uses genetic algorithm strategy to select probes that optimize the Mantel correlation among the samples. GA-Mantel was run 100 times and the probes that appeared in at least 30 of 100 solutions were picked. All analyses were performed using SAS Version 9.1 (SAS Institute).
Results
The effect of hypomethylating agents on FOXP3 expression in human and mouse CD4+CD25−FOXP3− T cells
Because the hypomethylating effect of Dec and AzaC can only occur in cells actively undergoing cellular division,29 we stimulated human CD4+CD25− T cells using anti-CD3/CD28 antibody-coated beads and hIL-2 (50 U/mL) before treatment with various concentrations of Dec and AzaC. Approximately 60% of Dec-treated CD4+CD25− T cells (dcTs) and only approximately 10% of pbsTs expressed FOXP3 (Figure 1A). Maximum FOXP3 expression was achieved after exposure to 5 to 10μM Dec for 4 days. However, cell counts significantly declined when T cells were exposed to concentrations of Dec in excess of 5μM (cell counts were 69.7% ± 14.1% of the 1μM Dec cell counts [n = 4]). Treating activated CD4+CD25− T cells or conventional T cells (Tconv; isolated using Miltenyi pan T-cell isolation kit) with 1μM Dec for 4 days optimized both cell expansion and FOXP3 expression. Real-time reverse transcription–polymerase chain reaction (RT-PCR) demonstrated levels of mRNA for FOXP3 that were comparable to those seen in bead-activated nTregs (10- to 12-fold increase above baseline; Figure 1B). Dec also induced FOXP3 expression in approximately 80% of bead-activated murine CD4+CD25− T cells (Figure 1A). Consistent with these results, both Dec and AzaC markedly increased GFP expression in activated CD4+CD25− T cells obtained from Foxp3-ires-GFP knock-in (KI) mice34 (Figure 1C). To determine the duration of FOXP3 expression in dcTs, we stained for intracellular FOXP3 daily for 7 days and found that approximately 50% of dcTs remained FOXP3+ at day 7 (Figure 1D). These results suggest that both hypomethylating agents induce both FOXP3 mRNA and protein expression in CD4+CD25− non-Tregs.
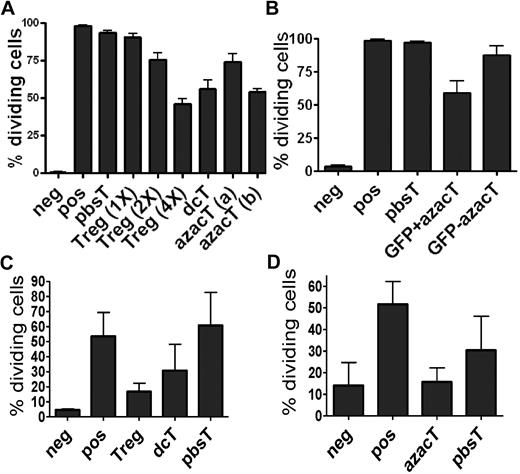
Effect of Dec on FOXP3 expression in anti-CD3/CD28 antibody coated bead-activated CD4+CD25− T cells. (A) Both human and mouse CD4+CD25− T cells express FOXP3 after Dec treatment in the presence of anti-CD3/CD28 antibody coated beads. (B) Real-time RT-PCR for human FOXP3 mRNA was performed in triplicate at various times after Dec treatment of activated T cells. Levels of mRNA for FOXP3 increase each day and are comparable to that seen in bead-activated Tregs (brown) by day 4. (C) CD4+CD25− T cells obtained from Foxp3-ires-GFP KI mice confirm induction of FOXP3 expression after Dec and AzaC treatment (indirectly measured as GFP expression). (D) DcT FOXP3 expression (blue) persists for at least 7 days, suggesting prolonged and stable expression of FOXP3 in vitro. Treg (red) is shown for comparison. A pool of 2 independent experiments.
Effect of Dec on FOXP3 expression in anti-CD3/CD28 antibody coated bead-activated CD4+CD25− T cells. (A) Both human and mouse CD4+CD25− T cells express FOXP3 after Dec treatment in the presence of anti-CD3/CD28 antibody coated beads. (B) Real-time RT-PCR for human FOXP3 mRNA was performed in triplicate at various times after Dec treatment of activated T cells. Levels of mRNA for FOXP3 increase each day and are comparable to that seen in bead-activated Tregs (brown) by day 4. (C) CD4+CD25− T cells obtained from Foxp3-ires-GFP KI mice confirm induction of FOXP3 expression after Dec and AzaC treatment (indirectly measured as GFP expression). (D) DcT FOXP3 expression (blue) persists for at least 7 days, suggesting prolonged and stable expression of FOXP3 in vitro. Treg (red) is shown for comparison. A pool of 2 independent experiments.
Suppressor function of hypomethylating agent-induced Tregs in vitro
We examined whether dcTs and AzaC-induced Tregs (azacTs) are suppressive in proliferation assays in which anti-CD3/CD28 beads were used as stimulators (Figure 2, supplemental Figures 1-2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Both dcTs and azacTs were able to function as suppressors, while pbsTs had no effect (Figure 2A, supplemental Figures 1-2). Using Foxp3-ires-GFP KI mice, we sorted FOXP3+ azacTs and FOXP3− azacTs based on GFP expression. Only GFP+ azacTs suppressed proliferation of Teffs in bead based activation assays, suggesting that the suppressor activity was derived from FOXP3+ (GFP+) cells (Figure 2B). Surprisingly, naive nTregs were less active than dcTs in suppressing the proliferation of CD4+CD25− Teffs at 1:1 nTreg:Teff ratios but could suppress proliferation of bead activated Teffs at higher Treg:Teff (2:1 or 4:1) ratios (Figure 2A). This unexpected result could be explained by the potent stimulatory effects of anti-CD3/CD28 beads on Teffs while naive nTregs are relatively slow to proliferate in response to beads and lack suppressive activity until they are activated. Thus, Teffs start to proliferate before nTregs while dcTs and azacTs are already activated before coculture with the Teffs. Supporting this idea, nTregs as well as dcTs and azacTs were able to function as suppressors in an in vitro MLR in the presence of stimulatory allogeneic antigen presenting cells (APCs; Figure 2C-D). During the 6 days of an MLR, nTregs become activated and suppress in these MLRs. In addition, when Tregs were activated with beads before the proliferation assay, we observed suppression although activated nTregs were still less suppressive than dcTs (supplemental Figure 2).
DcTs suppress the proliferation of Teffs. CFSE-based proliferation assays and MLR were performed with each population 2.5 × 104 per well in 96-well round-bottom plates. For transwell plate experiments, each population (1 × 105 per well) was incubated in 96-well flat-bottom transwell plates in the presence of beads as stimulators. The proliferation of CFSE-labeled CD4+CD25− T cells was analyzed after 3 days (cells with beads) or 6 days (cells with γ-irradiated APCs) on a FACScan cytometer (BD Biosciences). All cultures were evaluated in triplicate. Hypomethylating agent–treated cells suppress the proliferation of anti-CD3/CD28 antibody coated bead-activated Teffs (A-B) and allogeneic APC-activated Teffs (C-D). Treg (1×), (2×), and (4×) indicate the ratios of Treg:Teff = 1:1, 2:1, and 4:1, respectively. azacT (a) and (b) indicate that these azacTs were generated in the presence of AzaC 1μM and 2μM, respectivley. dcTs, azacTs, and pbsTs (right) were generated in the presence of hIL-2 (500 μ/mL; panel A). Only FACS purified GFP+ (thus, FOXP3+) and not GFP− CD4+ cells obtained from Foxp3-ires-GFP KI mice are suppressive (panel B). CD45.1 was used to gate on CFSE-labeled Teffs. dcT: Dec-treated T cells, pbsT: PBS-treated T cells, azacT: AzaC-treated T cells. Neg: negative control, CFSE-labeled Teffs alone; pos: CFSE-labeled Teffs with stimulators, anti-CD3/CD28 antibody coated beads or allogeneic APC; all others contain both CFSE-labeled Teffs and stimulators plus indicated cells such as nTregs, dcTs, pbsTs, or azacTs. GFP+ azacT: MoFlo sorted GFP+ cells after treatment of AzaC; GFP− azacT: MoFlo sorted GFP− cells after treatment of AzaC.
DcTs suppress the proliferation of Teffs. CFSE-based proliferation assays and MLR were performed with each population 2.5 × 104 per well in 96-well round-bottom plates. For transwell plate experiments, each population (1 × 105 per well) was incubated in 96-well flat-bottom transwell plates in the presence of beads as stimulators. The proliferation of CFSE-labeled CD4+CD25− T cells was analyzed after 3 days (cells with beads) or 6 days (cells with γ-irradiated APCs) on a FACScan cytometer (BD Biosciences). All cultures were evaluated in triplicate. Hypomethylating agent–treated cells suppress the proliferation of anti-CD3/CD28 antibody coated bead-activated Teffs (A-B) and allogeneic APC-activated Teffs (C-D). Treg (1×), (2×), and (4×) indicate the ratios of Treg:Teff = 1:1, 2:1, and 4:1, respectively. azacT (a) and (b) indicate that these azacTs were generated in the presence of AzaC 1μM and 2μM, respectivley. dcTs, azacTs, and pbsTs (right) were generated in the presence of hIL-2 (500 μ/mL; panel A). Only FACS purified GFP+ (thus, FOXP3+) and not GFP− CD4+ cells obtained from Foxp3-ires-GFP KI mice are suppressive (panel B). CD45.1 was used to gate on CFSE-labeled Teffs. dcT: Dec-treated T cells, pbsT: PBS-treated T cells, azacT: AzaC-treated T cells. Neg: negative control, CFSE-labeled Teffs alone; pos: CFSE-labeled Teffs with stimulators, anti-CD3/CD28 antibody coated beads or allogeneic APC; all others contain both CFSE-labeled Teffs and stimulators plus indicated cells such as nTregs, dcTs, pbsTs, or azacTs. GFP+ azacT: MoFlo sorted GFP+ cells after treatment of AzaC; GFP− azacT: MoFlo sorted GFP− cells after treatment of AzaC.
The strong suppressor activity after Dec or AzaC treatment may be due to their effects on altering both specific gene and global gene expression profiles. It is, therefore, likely that Dec and AzaC induce not only Foxp3, but other genes that are critically involved in the suppressor function of nTregs, perhaps even more dramatically than in resting or activated nTregs, thereby making dcTs more potent than nTregs in these in vitro bead based activation assays. nTregs appear to have multiple mechanisms of suppressor function.35-44 In some cases nTregs use CD25 or IL-35 as mediators of suppression while, in others, they use galectin-1, PRF1/GZMB, or CTLA-4.35,36,40-44 They even secrete immunosuppressive cytokines, such as TGF-beta and IL-10, especially in the case of adaptive Tregs.37-39 By comparing the gene expression profiles of dcTs and nTregs, we found that many of the candidate genes associated with Treg function (IL-35, CD25, galectin-1, Prf1, GzmB, TGF-beta, and IL-10) with the exception of CTLA-4 were expressed at higher levels in dcTs than in naive or activated nTregs (supplemental Table 1). These data suggest that hypomethylating agents modulate genes that have been shown to be associated with the suppressive phenotype of Tregs.
In vivo suppressor function of hypomethylating agent-induced Tregs generated in vitro
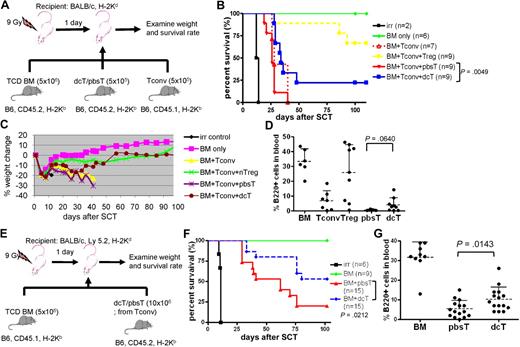
We next examined whether dcTs and azacTs are able to suppress GVHD in an allogeneic SCT model. Lethally irradiated Balb/c mice underwent a transplantation with TCD BM (CD45.2) and Tconv (CD45.1) isolated from B6 mice along with dcTs, azacTs, or pbsTs generated from B6 (CD45.2; Figure 3A). Mice that underwent a transplantation with dcTs had complete donor chimerism, improved survival, and significantly less weight loss compared with mice that received pbsTs (Figure 3B-C and supplemental Figure 3). These results are consistent with less clinical GVHD in the dcT group. Similar results were observed when azacTs were tested, resulting in improved survival compared with pbsTs (supplemental Figure 4). These data suggest that both dcTs and azacTs can function as suppressor cells in vivo. The mean percentages of donor-derived B cells in the peripheral blood were also higher in the dcT group than in the pbsT group (Figure 3D). Despite the reduced signs of GVHD seen in the dcT group compared with the pbsT group, the overall survival of the dcT group was inferior to that of the nTreg group. One explanation is that the percentage of FOXP3+ cells in dcTs is only 60% to 80% compared with the over 90% Treg group. Therefore, dcTs generated in vitro contain significant FOXP3− CD4+CD25+ activated Teffs (ie, FOXP3− dcTs) which, like Tconv, may both promote engraftment and GVHD in murine transplant recipients. We therefore tested whether dcTs generated from Tconv, as opposed to purified CD4+CD25− T cells, could promote engraftment (by FOXP3− dcTs) and mitigate GVHD (by FOXP3+ dcTs) in an allogeneic SCT (Figure 3E). We found that mice that received these dcTs demonstrated improved survival compared with mice that received pbsTs (Figure 3F). In addition, mice that received Tconv-generated dcTs demonstrated complete donor chimerism and significantly higher numbers of donor B cells than mice that received pbsTs (Figure 3G and supplemental Figure 5). These data are consistent with less clinical GVHD in the dcT group.
DcTs mitigate GVHD. (A) Schema of the experiments. 9 Gy total-body irradiation was used to condition recipient mice (Balb/c). B6 mice (CD45.2) TCD BM (5 × 106 cells) were used as a stem cell source. To induce GVHD, 5 × 105 B6 Tconv (CD45.1) was infused along with donor TCD BM. To test suppressor function of dcTs, 5 × 105 B6 CD4+CD25+ nTregs (for control), pbsTs or dcTs (all CD45.2) generated from CD4+CD25− T cells were injected with TCD BM and Tconv. (B-D) Mice that underwent a transplantation with dcTs show significantly higher survival rate (B) and less weight loss (C) than mice infused with pbsTs and a trend toward increase of B cells (D) analyzed 1 month after transplantation. A pool of 2 independent experiments. (E) Schema of the experiments. B6 mice (CD45.1) TCD BM (5 × 106 cells) were used as a stem cell source. A total of 10 × 106 pbsTs/dcTs generated from Tconv (B6, CD45.2) were given. (F-G) Mice that underwent a transplantation with dcTs show significantly higher survival rate (F) and more B cells (G) than mice that received pbsTs. A pool of 3 independent experiments analyzed 1 month after transplantation.
DcTs mitigate GVHD. (A) Schema of the experiments. 9 Gy total-body irradiation was used to condition recipient mice (Balb/c). B6 mice (CD45.2) TCD BM (5 × 106 cells) were used as a stem cell source. To induce GVHD, 5 × 105 B6 Tconv (CD45.1) was infused along with donor TCD BM. To test suppressor function of dcTs, 5 × 105 B6 CD4+CD25+ nTregs (for control), pbsTs or dcTs (all CD45.2) generated from CD4+CD25− T cells were injected with TCD BM and Tconv. (B-D) Mice that underwent a transplantation with dcTs show significantly higher survival rate (B) and less weight loss (C) than mice infused with pbsTs and a trend toward increase of B cells (D) analyzed 1 month after transplantation. A pool of 2 independent experiments. (E) Schema of the experiments. B6 mice (CD45.1) TCD BM (5 × 106 cells) were used as a stem cell source. A total of 10 × 106 pbsTs/dcTs generated from Tconv (B6, CD45.2) were given. (F-G) Mice that underwent a transplantation with dcTs show significantly higher survival rate (F) and more B cells (G) than mice that received pbsTs. A pool of 3 independent experiments analyzed 1 month after transplantation.
The effect of in vivo treatment of mice after allogeneic SCT with hypomethylating agents: impact on engraftment, GVHD, and immune reconstitution
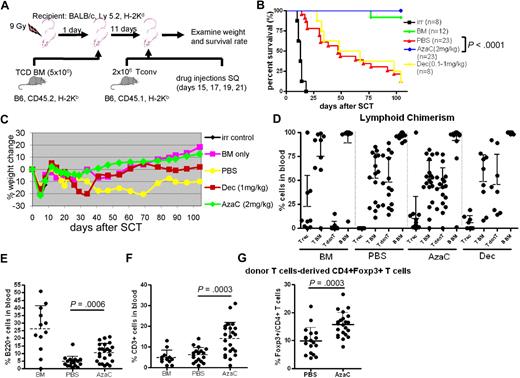
We examined whether in vivo treatment of mice with hypomethylating drugs after transplantation could mitigate GVHD by increasing FOXP3+ Tregs in murine allogeneic transplantation models (Figure 4A). To minimize the death related to myelosuppression of these hypomethylating agents when given immediately after transplantation we first infused T cell–depleted allogeneic BM (5 × 106; CD45.2) after TBI (900 cGy) on day 0. After recovery of blood counts mice were then infused with 2 × 106 major histocompatibility complex (MHC) mismatched Tconv on day +11. Mice were treated with PBS, Dec (1 mg/kg) or AzaC (2 mg/kg) subcutaneously on days +15, +17, +19, and +21. Despite delay in treatment with Dec, we observed that the survival rate of mice treated in vivo with Dec after allogeneic SCT was not different from mice treated after transplantation with PBS due to the excessive myelosuppressive effects of Dec (all deaths were associated with pancytopenia; Figure 4B). In sharp contrast, AzaC treatment of mice that underwent a transplantation with TCD BM plus 2 × 106 MHC mismatched Tconv cells resulted in no detectable GVHD-related symptoms, such as weight loss, ruffled fur, or diarrhea (Figure 4B-C). Most importantly, 100% of mice (23/23) that received posttransplantation AzaC and donor T cells in vivo survived with donor engraftment (20/23 showed ≥ 85% donor cells) and no GVHD similar to those mice that received only TCD BM without donor T cells and dramatically different from those mice that received donor T cells without AzaC (3/23 survived; Figure 4B-D). We also found that the AzaC group had high percentages of donor CD3+ T cells and B220+ B cells after transplantation, which is consistent with less clinical GVHD (Figure 4E-F). Significant increase of FOXP3+ Tregs in the peripheral blood was also noted in mice treated in vivo with AzaC after transplantation, suggesting that the suppression of GVHD may be mediated, at least in part, by AzaC-induced peripheral conversion of FOXP3− donor T cells to FOXP3+ Tregs in vivo (Figure 4G). Although it is possible that AzaC promoted the expansion of infused donor nTregs resulting in reduced GVHD, mice that received Treg-depleted Tconv (> 98% FOXP3− Tconv) and were treated with AzaC in vivo also had dramatically reduced GVHD (supplemental Figure 6).
AzaC treatment of mice that underwent a transplantation with delayed allogeneic T cells mitigates GVHD. (A) Schema of the experiments. B6 mice (CD45.2) TCD BM (5 × 106 cells) were used as a stem cell source. To induce GVHD, 2 × 106 Tconv (B6, CD45.1) were given on day 11 after SCT followed by the treatment with AzaC/Dec/PBS (every other day; 4 doses) starting on day 15 after SCT. (B-F) Mice treated with AzaC (2 mg/kg) show significantly higher survival rate (B), less weight loss (C), and more B cells (E) and T cells (F) with donor engraftment (D) than mice infused with pbsTs. (G) AzaC group also show increased Treg population in peripheral blood. T rec: T cells from recipient; T BM: T cells from donor BM; T donT: T cells from donor T cells; B BM: B cells from donor BM. (D-G) Analyzed 1 month after transplantation. A pool of 4 independent experiments.
AzaC treatment of mice that underwent a transplantation with delayed allogeneic T cells mitigates GVHD. (A) Schema of the experiments. B6 mice (CD45.2) TCD BM (5 × 106 cells) were used as a stem cell source. To induce GVHD, 2 × 106 Tconv (B6, CD45.1) were given on day 11 after SCT followed by the treatment with AzaC/Dec/PBS (every other day; 4 doses) starting on day 15 after SCT. (B-F) Mice treated with AzaC (2 mg/kg) show significantly higher survival rate (B), less weight loss (C), and more B cells (E) and T cells (F) with donor engraftment (D) than mice infused with pbsTs. (G) AzaC group also show increased Treg population in peripheral blood. T rec: T cells from recipient; T BM: T cells from donor BM; T donT: T cells from donor T cells; B BM: B cells from donor BM. (D-G) Analyzed 1 month after transplantation. A pool of 4 independent experiments.
Recently, Perez-Simon and colleagues proposed that AzaC might directly suppress the proliferation of Tconv and thus GVHD based on in vitro cell culture data in which more than 99% of Tconv were blocked at G0/G1 phase after CD3/CD28 bead activation in the presence of AzaC.45 To determine the impact of AzaC in vivo, Tconv were first labeled with CFSE and then infused on day +11 after transplantation. Mice were treated in vivo with either PBS, or AzaC as described above. Peripheral blood was obtained on day +19 and FACS gated on donor Tconv (H-2Kb; CD45.1) was performed. Surprisingly, we observed that Tconv proliferated robustly in vivo after AzaC treatment (Figure 5). Moreover, all of the FOXP3+ Tregs were derived from donor Tconv that had divided the most in vivo (Figure 5). These results are consistent with the critical role of cellular proliferation on the biologic activities of both Dec and AzaC. Therefore, the modest reduction of proliferation of donor Tconv in transplant recipients treated with AzaC could be explained by a direct effect on Tconv proliferation or more likely on the peripheral conversion of FOXP3− Tconv to FOXP3+ Tregs. These in vivo generated “azacTs” could then suppress the proliferation of Tconv, thus limiting clinical GVHD while maintaining a potent GVL effect (see next paragraph).
AzaC increase FOXP3+ Tregs that may inhibit the proliferation of allogeneic Tconv in vivo. In vivo AzaC treatment (blue) attenuates the proliferation of allogeneic Tconv compared with the PBS control (red, left panel; peripheral blood on day 19 after SCT, gated on CD45.1+ donor T cells). This inhibited proliferation of Tconv is likely to be mediated by FOXP3+ Tregs induced by AzaC (middle and right; splenocytes on day 19 after SCT, gated on CD45.1+ donor T cells). One representative from each group with identical results is shown. AzaC (n = 4), PBS (n = 2).
AzaC increase FOXP3+ Tregs that may inhibit the proliferation of allogeneic Tconv in vivo. In vivo AzaC treatment (blue) attenuates the proliferation of allogeneic Tconv compared with the PBS control (red, left panel; peripheral blood on day 19 after SCT, gated on CD45.1+ donor T cells). This inhibited proliferation of Tconv is likely to be mediated by FOXP3+ Tregs induced by AzaC (middle and right; splenocytes on day 19 after SCT, gated on CD45.1+ donor T cells). One representative from each group with identical results is shown. AzaC (n = 4), PBS (n = 2).
The effect of in vivo treatment of mice after allogeneic SCT with hypomethylating agents: impact on GVL
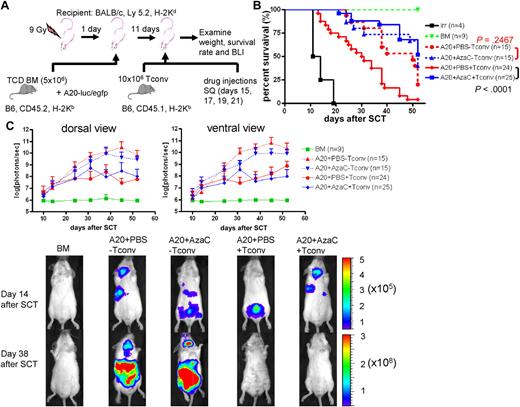
It has been shown that nTregs preferentially suppress GVHD while maintaining GVL. Thus, we examined whether AzaC injection after allogeneic SCT results in similar effects. Using a well-established GVL model involving luciferase-expressing murine A20 leukemia cells, we tested the clearance of leukemia cells after allogeneic SCT (PBS vs AzaC treatment after transplantation in vivo) using in vivo bioluminescent imaging (BLI)31 (Figure 6A). We tested 2 different doses of Tconv: 2 × 106 and 10 × 106. While both doses preserved GVL activity after AzaC treatment, 10 × 106 Tconv induced a more robust GVHD only in those mice treated with PBS (supplemental Figure 7). Thus, we used 10 × 106 Tconv for all the following GVL experiments. While AzaC treatment had no effect on the growth of A20 leukemia cells in vivo (A20+PBS-Tconv vs A20+AzaC−Tconv; Figure 6B-C), the AzaC group (A20+AzaC+Tconv) had a significantly lower leukemic burden and percentage of mice with leukemia than the control group (A20+AzaC−Tconv) and comparable to the PBS group and a significantly better survival rate than the PBS group (A20+PBS+Tconv), suggesting that AzaC treatment of mice after allogeneic SCT preferentially suppresses GVHD while maintaining GVL (Figure 6B-C, supplemental Figures 7-8).
AzaC treatment of mice that underwent a transplantation with delayed allogeneic T cells mitigates GVHD while preserving GVL. (A) Schema of the experiments. B6 mice (CD45.2) TCD BM (5 × 106 cells) were used as a stem cell source. To induce GVHD, 10 × 106 Tconv (B6, CD45.1) were given on day 11 after SCT followed by the treatment with AzaC or PBS (every other day; 4 doses) starting on day 15 after SCT. For examination of GVL effect, 1 × 104 A20-luc/egfp leukemic cells were given along with TCD BM. (B-C) Mice treated with AzaC show significantly higher survival rate (B) and lower leukemic burden (C). Y axis in top panels indicates photon flux (photons/sec) in log scale measured from the dorsal and the ventral view with a region of interest drawn over the entire body of each mouse. Actual images of 1 representative mouse from each group are shown in bottom panels (scale: photons/sec/cm2/sr). A pool of 3 independent experiments.
AzaC treatment of mice that underwent a transplantation with delayed allogeneic T cells mitigates GVHD while preserving GVL. (A) Schema of the experiments. B6 mice (CD45.2) TCD BM (5 × 106 cells) were used as a stem cell source. To induce GVHD, 10 × 106 Tconv (B6, CD45.1) were given on day 11 after SCT followed by the treatment with AzaC or PBS (every other day; 4 doses) starting on day 15 after SCT. For examination of GVL effect, 1 × 104 A20-luc/egfp leukemic cells were given along with TCD BM. (B-C) Mice treated with AzaC show significantly higher survival rate (B) and lower leukemic burden (C). Y axis in top panels indicates photon flux (photons/sec) in log scale measured from the dorsal and the ventral view with a region of interest drawn over the entire body of each mouse. Actual images of 1 representative mouse from each group are shown in bottom panels (scale: photons/sec/cm2/sr). A pool of 3 independent experiments.
The mechanisms of hypomethylating agent-mediated induction of Tregs
Two appealing models of how Tregs suppress the proliferation of Teffs are (1) a cell-contact (or close proximity) dependent mechanism via perforin 1 (PRF1) and granzyme B (GZMB), IL-35, cell surface-bound TGF-β or Galectin-1, and CD25, and (2) a cell-contact independent mechanism mediated by secreted immunosuppressive cytokines, such as TGF-β and IL-10.35-44 To determine whether or not dcTs function in a cell-contact dependent fashion, we performed proliferation assays using coculture (direct contact) and transwell plates (cell contact-independent). As shown in supplemental Figure 9, coculture of dcTs and Teffs was necessary for suppression, suggesting that dcTs function in a cell-contact or close proximity dependent manner. Next, we determined whether suppressor function of dcTs requires PRF1 and GZMB using dcTs generated from Prf1 and GzmB deficient (KO) mice.46,47 DcTs from Prf1 KO and GzmB KO mice were highly suppressive, suggesting that these proteins are not necessary for the suppressor function of dcTs, although PRF1 might be partially required (Figure 7A).
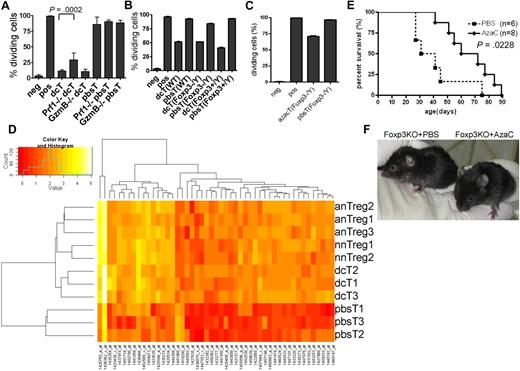
Mechanism of hypomethylating agent-induced generation of Tregs. (A) Perforin (Prf1) is partially required for the suppressor function of dcT. DcTs from both WT and granzyme B (GzmB)–deficient mice were equally suppressive while those from Prf1-deficient mice were significantly less suppressive. A pool of 2 independent experiments. (B-C) The impact of dcTs (B) and azacTs (C) from WT, Foxp3 heterozygote (Foxp3+/Y) and Foxp3 deficient (Foxp3−/Y) mice on Teffs were compared in proliferation assays. They are equally suppressive regardless of their origins. Neg: negative control, CFSE-labeled Teffs alone; pos: CFSE-labeled Teffs with stimulators, anti-CD3/CD28 antibody coated beads or allogeneic APC; all others contain both CFSE-labeled Teffs and stimulators plus indicated cells. (D) GA-Mantel33 analysis was run 100 times. Forty-nine probes appeared in at least 30 of 100 solutions (Mantel correlation = 0.97). Foxp3 is the most frequently selected probe. Heat map shows these 49 probes that separate the 3 Treg groups from the pbsT group. nnTreg: naive nTregs (n = 2), anTreg: activated nTregs (n = 3), dcT (n = 3), pbsT (n = 3). (E) AzaC treatment prolongs survival of Foxp3 deficient mice. A treatment of 2 mg/kg AzaC was given every other day (4 doses) starting at 16 to 26 days of age. A pool of 4 independent experiments. (F) Shown are 41-day-old Foxp3 KO mice treated with PBS (left) and AzaC (right); 25% (n = 8) had WT-looking ears.
Mechanism of hypomethylating agent-induced generation of Tregs. (A) Perforin (Prf1) is partially required for the suppressor function of dcT. DcTs from both WT and granzyme B (GzmB)–deficient mice were equally suppressive while those from Prf1-deficient mice were significantly less suppressive. A pool of 2 independent experiments. (B-C) The impact of dcTs (B) and azacTs (C) from WT, Foxp3 heterozygote (Foxp3+/Y) and Foxp3 deficient (Foxp3−/Y) mice on Teffs were compared in proliferation assays. They are equally suppressive regardless of their origins. Neg: negative control, CFSE-labeled Teffs alone; pos: CFSE-labeled Teffs with stimulators, anti-CD3/CD28 antibody coated beads or allogeneic APC; all others contain both CFSE-labeled Teffs and stimulators plus indicated cells. (D) GA-Mantel33 analysis was run 100 times. Forty-nine probes appeared in at least 30 of 100 solutions (Mantel correlation = 0.97). Foxp3 is the most frequently selected probe. Heat map shows these 49 probes that separate the 3 Treg groups from the pbsT group. nnTreg: naive nTregs (n = 2), anTreg: activated nTregs (n = 3), dcT (n = 3), pbsT (n = 3). (E) AzaC treatment prolongs survival of Foxp3 deficient mice. A treatment of 2 mg/kg AzaC was given every other day (4 doses) starting at 16 to 26 days of age. A pool of 4 independent experiments. (F) Shown are 41-day-old Foxp3 KO mice treated with PBS (left) and AzaC (right); 25% (n = 8) had WT-looking ears.
Because Dec and AzaC treatment leads to global hypomethylation, it raised the question of whether dcTs and azacTs function in a FOXP3-dependent manner. We observed that the dcTs from the Foxp3 KO mice5 (Foxp3−/Y) were as suppressive as dcTs from the Foxp3 wild-type (WT) littermate controls (Foxp3+/Y) and WT B6 mice (Figure 7B). We also observed similar results with azacTs generated from Foxp3 KO mice (Figure 7C). These results suggest that the suppressor function of dcTs and azacTs does not require FOXP3 expression and that the treatment with Dec and AzaC induces the expression of not only FOXP3, but also modulates the expression of other candidate genes that are necessary for the suppressor function of azacTs and dcTs. These genes may be independent or downstream targets of FOXP3 and regulated, like Foxp3, by DNA methylation. Therefore, we performed genome-wide RNA profiling analyses using non-amplified total RNAs in an effort to identify candidate genes that are regulated by hypomethylating agents and potentially mediate the observed suppressive function of nTregs, dcTs, and azacTs. Using GA-Mantel analysis,33 we identified 49 probes/genes that were expressed in a similar fashion within the 3 Treg groups (naive nTregs, activated nTregs, and dcTs) but differently in the pbsT control group (Figure 7D and supplemental Table 2). As we expected, Foxp3 was one of these genes. Among these 48 candidate genes, 46 were up-regulated and 2 down-regulated in the Treg groups.
Effect of AzaC on Foxp3 KO mice
Because the suppressor function of these hypomethylating agent-induced Tregs is independent of FOXP3 and since AzaC injection in vivo postallogeneic SCT mitigates GVHD by increasing Tregs, we hypothesized that injection of AzaC might suppress autoimmuity and increase survival of Foxp3 KO mice by converting auto-reactive T cells into Foxp3-independent Tregs. We observed that injections of AzaC shortly after birth (days 16-26) significantly improved survival of these mice compared with Foxp3 KO mice injected with PBS control (Figure 7E). In addition, AzaC suppressed autoimmunity, based on the observation that the AzaC group had normal looking ears while the PBS group had small, scaly, and thick ears, which is one of the features of Foxp3 KO mice (Figure 7F).
Discussion
In this report, we describe a novel and simple approach to limit GVHD while maintaining GVL by generating Tregs from alloreactive donor T cells in vivo using AzaC. This approach may help to overcome the current obstacles for the routine use of nTregs in the human clinical trials including limiting numbers of nTregs in peripheral blood, loss of suppressor properties after in vitro expansion, and lack of cell surface markers necessary for efficient affinity purification.5,7-9,20-22
The Foxp3-independence of dcTs and azacTs suggests that Foxp3 and the genes responsible for the suppressor function of Tregs are probably regulated by the same mechanism, that is, DNA methylation. Thus, although Dec and AzaC can directly induce FOXP3 expression, they can also modulate the expression of FOXP3 target genes required for the suppressor function of Tregs. This suggests that these genes as well as Foxp3 remain hypomethylated in Tregs, but methylated in non-Tregs. A recent report demonstrates that Foxp3 is not required for the development of nTregs, but for the maintenance of nTreg suppressor properties by maintaining the expression of Foxp3 target genes.48 This observation suggests that Foxp3 might be involved in maintaining hypomethylation status of these Foxp3 target genes or is essential for the increased or continued expression of these candidate genes. These data may suggest why AzaC treatment of Foxp3 KO mice did not permanently rescue them in the absence of continued expression of Foxp3. In an elegant system in which the Foxp3 gene has been replaced with GFP, the same group showed that GFP+ (FOXP3−) cells contain gene expression profiles similar to nTregs.48 Together with our data this observation strongly suggests that there is a common regulatory mechanism that controls the DNA methylation status of both Foxp3 and its target genes.
In an effort to identify these target genes, we found 48 candidate genes that are differentially expressed in the Treg groups comparing to the pbsT group. Since their functions in Tregs are currently unknown (with the exception of Foxp3), it will be of interest to determine whether one or more of these genes are responsible for the suppressor function of Tregs and if their expression can be modulated by hypomethylating agents in CD4+ cells from both WT and Foxp3 KO mice. Finally, it would be of interest to determine whether any of these gene products are expressed on the cell surface and could potentially be used for purification of nTregs, ex vivo–expanded nTregs, or hypomethylating agent–induced Tregs.
Although the suppressor function of dcTs and azacTs are Foxp3-independent, FOXP3 expression is currently the only surrogate marker of the hypomethylation status of those genes critical in the generation of Tregs. As shown in Figure 2B, only GFP+ azacTs were suppressive. It is possible that GFP− azacTs might be nonproliferating cells limiting the ability of AzaC to reduce the promoter methylation status of critical Treg-dependent genes in these cells since AzaC can incorporate only into replicating DNA.26-29 In addition to the direct hypomethylation of target genes by Dec and AzaC, it is also possible that Dec and AzaC might alter miRNA expression, thus altering expression of Foxp3 target genes and their suppressor functions.49 In addition, Dec and AzaC might have other unknown functions. It has been recently suggested that AzaC may have indirect effects on gene expression via DNA damage/stress response signaling pathways.50,51
It is possible that AzaC inhibits activation and proliferation of alloreactive donor T cells, thereby decreasing GVHD as proposed by Perez-Simon and colleagues based on their in vitro experiments.45 According to our CFSE-based in vivo experiments (Figure 5) and GVL experiments (Figure 6), however, it is not likely that AzaC simply eliminates or blocks the proliferation of allogeneic Tconv. Rather, it is more likely that AzaC treatment suppresses GVHD while maintaining a potent GVL effect by peripheral conversion of donor T cells into FOXP3+ Tregs. Nonetheless, we do not exclude the possibility that AzaC indirectly attenuates the proliferation of Tconv by decreasing proinflammatory cytokines. Indeed, we observed reduced levels of serum IFN-γ, IL-6, and IL-10 in the AzaC group (supplemental Figure 10; high serum IL-10 levels and high dose of IL-10 administration are associated with increased risks of GVHD in humans52,53 and mice,54 respectively55 ). It is not clear at present whether AzaC mitigates GVHD via direct effects on Tconv or via reducing the production of inflammatory cytokines, or both. Therefore, future studies will focus on studies determining the molecular mechanisms by which AzaC reduces reduction of GVHD while maintaining a potent GVL response.
While Noelle and colleagues suggest that the suppressor function of mouse nTregs is independent of Prf1,41 our data are consistent with previous reports on both human and mouse nTregs.42,56,57 Our data suggest that Prf1 may play at least a partial role in suppressive function of both nTregs and dcTs. Our experiments further demonstrate that dcTs, like nTregs, require direct contact or close proximity for their suppressive effects. In addition, although GzmB appears to be necessary for a full suppressor function of mouse nTregs when they regulate NK cells and B cells,56,57 our data demonstrating that the suppressor function of dcTs is independent of GzmB are consistent with a recent report on mouse nTregs in the allogeneic transplantation setting.58 Finally, we have demonstrated that although dcTs appear to be equally suppressive to nTregs in vitro, they are significantly less suppressive than nTregs when generated in vitro and infused in vivo (Figure 3B). Similar results are seen when purified nTregs have been activated/expanded in vitro and tested in vivo.13,16 The reduction of in vivo suppressive activity of dcTs might be due to reduced survival in vivo after strong ex vivo activation/expansion with low concentrations of IL-2 (10 U/mL). Alternatively, ex vivo–activated dcTs (like ex vivo–expanded nTregs) may have significant alterations in trafficking in vivo, thus limiting optimal interactions and direct contact with Teffs at sites of Teff expansion in vivo.
In conclusion, AzaC treatment of allo-transplanted mice mitigates deleterious GVHD while preserving beneficial GVL. The beneficial effect of AzaC on GVHD in these preclinical studies has only been tested when given within days after the infusion of alloreactive donor T cells. It is conceivable that delaying the infusions of AzaC may have an impact on both GVHD and GVL. Thus the effect of altering the timing of AzaC treatment will need to be explored in the future. Our studies have also demonstrated that AzaC treatment in vivo also suppresses autoimmunity in Foxp3 KO mice and prolongs their survival. Thus, the administration of hypomethylating agents such as AzaC after transplantation in humans undergoing allogeneic SCT may provide a simple and relatively nontoxic approach to limiting GVHD while preserving the GVL and engraftment potential of donor T cells. These studies also provide insights into alternative pathways and candidate genes (in addition to Foxp3), which both may mediate the suppressive function of regulatory T cells and, like Foxp3, are regulated by DNA methylation.
Presented in abstract form at the 50th annual meeting of the American Society of Hematology, San Francisco, CA, December 8, 2008.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Tim Ley and Sheng Cai for providing Foxp3-GFP KI, Prf1 KO, and GzmB KO mice and Chyi Hsieh for Foxp3 KO mice. We thank Le'erin Voss, Sandeep Sodhi, and Mark Needles for assisting our research as summer students, and Mark Schroeder and Linda Eissenberg for critical reading the manuscript. J.F.D. is supported by the National Cancer Institute (R01 CA83845; and R21 grants CA110489, CA132269, CA141523 P01 CA101937, and P50 CA94056). J.C. is supported by the Bryan Thomas Campbell Foundation. D.R.P.-W. is supported by P50 CA94056.
National Institutes of Health
Authorship
Contribution: J.C. and J.F.D. designed and analyzed the experiments and wrote the paper; J.C. and J.R. performed the animal study; J.C., J.L.P., and D.R.P.-W. carried out BLI; J.C. and M.H. conducted real-time RT-PCR; W.D.S. and E.D. analyzed RNA profiles; and all authors discussed the results and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John F. DiPersio, MD, PhD, Division of Oncology, Washington University School of Medicine, 660 S Euclid Ave, Campus Box 8007, St Louis, MO 63110; e-mail: jdipersi@dom.wustl.edu.