Abstract
Combination tacrolimus and sirolimus graft-versus-host disease (GVHD) prophylaxis for allogeneic transplant in patients conditioned with a fractionated total body irradiation–based regimen has shown encouraging results. We studied this prophylaxis combination in 85 patients receiving a matched-sibling transplant conditioned with 3 different regimens:fludarabine-melphalan (n = 46); total body irradiation–etoposide (n = 28), and busulfan-cyclophosphamide (n = 11). The conditioning regimens were completed on day −4. Sirolimus and tacrolimus were started on day −3 to avoid overlap with conditioning therapy. All patients engrafted, with a median time to neutrophil engraftment of 15 days. The cumulative incidence of acute GVHD grades II to IV and III to IV was 43% and 19%, respectively, with no significant difference by conditioning regimen. The 2-year cumulative incidence of chronic GVHD was 46%. With a median follow-up of 26 months, disease-free survival was 58% and overall survival, 66%. The day-100 and 2-year nonrelapse mortality was 4.8% and 10.2%, respectively. The overall incidence of thrombotic microangiopathy was 19%, and it was significantly higher with busulfan/cyclophosphamide (55%, P = .005). Tacrolimus plus sirolimus is an effective combination for acute GVHD prophylaxis and is associated with very low nonrelapse mortality. Thrombotic microangiopathy is a significant complication with this regimen, particularly in patients receiving busulfan/cyclophosphamide.
Introduction
Graft-versus-host disease (GVHD) remains one of the most challenging obstacles to successful allogeneic hematopoietic cell transplantation (HCT). Standard prophylactic regimens with tacrolimus/methotrexate or cyclosporine/methotrexate are associated with acute GVHD in close to half of sibling transplants.1
Sirolimus, originally named rapamycin, binds to the same family of intracellular FK-506 (tacrolimus)–binding proteins (FKBP12 and others) at a site distinct from tacrolimus. Sirolimus-FKBP complexes inhibit the mammalian target of rapamycin (mTOR), a kinase that regulates cell cycle entry in response to interleukin 2 (IL-2) signaling and other cellular functions. Cell cycle entry in the presence of sirolimus induces T-cell apoptosis, and deprivation of IL-2 signaling renders antigen-activated T cells unresponsive. Both mechanisms are thought to contribute to T-cell immunologic tolerance.2 Sirolimus has been widely used as an immunosuppressant in solid organ transplantation to prevent immune-mediated graft rejection3-5 and has been evaluated for treatment of acute GVHD6 and chronic GVHD.7
Recently, the novel combination of tacrolimus and sirolimus was evaluated by researchers at the Dana-Farber Cancer Institute (DFCI) in a cohort of 30 patients who received matched-related peripheral blood stem cells and were conditioned with fractionated total body irradiation (TBI) and cyclophosphamide (Cy).8,9 The extended results of 53 matched-related HCTs were very promising in that the cumulative incidence of grade II to IV acute GVHD was 19%, and only 3 patients developed grade III to IV acute GVHD.9 The nonrelapse mortality (NRM) rates were also encouraging; the day-30 and day-100 rates were 0% and 5%, respectively.9 Another positive outcome, seen in this study and others, was that the use of sirolimus was found to be associated with reductions in cytomegalovirus (CMV) viremia10 and the incidence/severity of oral mucositis.11 However, sirolimus is associated with an increased risk of thrombotic microangiopathy (TMA), with a rate of 10.8% in the sirolimus group and 4.2% in the nonsirolimus group (odds ratio, 2.79, P = .03) in a retrospective analysis of myeloablative allogeneic HCT recipients.12 Another recent study showed an increase in veno-occlusive disease (VOD) associated with sirolimus use, particularly when methotrexate was added to the GVHD prophylaxis, or busulfan (Bu)/Cy was used in conditioning.13
Thus far data have been reported exclusively from DFCI except for a recent publication from the Fred Hutchinson Cancer Center (FHCC) evaluating sirolimus combined with either tacrolimus or cyclosporine in unrelated donor HCT (n = 26) with an unexpectedly high incidence of acute GVHD (77%).14 Due to toxicity, administration of sirolimus was discontinued earlier than planned in 11 patients, but after the onset of GVHD. Possible explanations for the discrepant results between DFCI and FHCC may include differential timing of immunosuppression, use of cyclosporine instead of tacrolimus in some cases, and use of Bu/Cy in some cases.
In this prospective pilot/phase II trial, conducted at City of Hope, we sought to determine the efficacy and safety of sirolimus combined with tacrolimus for 3 different conditioning regimens: fludarabine-melphalan (Flu/Mel), TBI-etoposide (TBI/VP16), and Bu/Cy. The secondary objectives of this study included an evaluation of risk factors associated with acute GVHD and TMA. This protocol extended the DFCI experience before the phase III trial sponsored by the Blood and Marrow Transplant Clinical Trial Network (BMT CTN). We designed the study to complete the conditioning regimen on day −4, before starting the immunosuppression on day −3 to prevent possible drug interactions between conditioning chemotherapeutic drugs and tacrolimus/sirolimus.
Methods
Clinical trial
This phase II study, Institutional ReviewBoard 04052, was approved by the City of Hope Institutional ReviewBoard. The primary endpoints were toxicity (including TMA) and incidence/severity of acute GVHD; secondary endpoints included incidence of chronic GVHD and survival at 100 days, 1 year, and 2 years. Acute and chronic GVHD events were graded according to established criteria.15,16 Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE v3.0).17 TMA was defined as the simultaneous occurrence of schistocytosis, increased lactate dehydrogenase, and persistent thrombocytopenia (below 50 000/μL). Patients were eligible if they had a matched sibling donor and adequate organ function, defined as creatinine clearance more than 50 mL/minute, pulmonary diffusion capacity (DLCO) of more than 50%, and cardiac ejection fraction of more than 50%. Conditioning regimen selection followed City of Hope standard treatment/practice guidelines, using TBI/VP16 for patients younger than 50 years with acute leukemia in first or subsequent remission, Flu/Mel for patients older than 50 years or with comorbid conditions, and Bu/Cy for patients with myeloproliferative disorders in chronic phase or acute leukemia and myelodysplastic syndrome with contraindication to TBI. Peripheral stem cell donors were mobilized with granulocyte colony-stimulating factor 10 μg/kg for 4 days, to a targeted CD34+ cell dose of 2 to 5 × 106/kg. The day of stem cell infusion was defined as day 0. Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of 500 cells/μL.
GVHD prophylaxis
The GVHD prophylaxis was administered according to published reports,8 as follows: sirolimus 12 mg by mouth on day −3 (loading dose), followed by 4 mg orally daily, with the 4-mg dose being adjusted as necessary to maintain serum levels between 3 and 12 ng/mL by high performance liquid chromatography (HPLC); tacrolimus 0.02 mg/kg intravenously daily starting on day −3, switched to an equivalent oral dose when oral intake was adequate, to maintain target serum levels of 5 to 10 ng/mL. Tacrolimus and sirolimus levels were measured at least weekly until around day 100, and the dose was adjusted for the target levels and for clinical toxicity. Tapering of immunosuppression was to start around day 100; tapering was allowed earlier for relapsed malignancy.
Conditioning regimen
For TBI/VP16, 1320 cGy in 11 fractions was given from day −8 to day −5, followed by etoposide at 60 mg/kg of adjusted ideal body weight on day −4. For Flu/Mel, fludarabine was given intravenously at 25 mg/m2 per day for 5 days, from day −9 to day −5, and melphalan was given intravenously at 140 mg/m2 of adjusted ideal body weight on day −4. For Bu/Cy, busulfan was given intravenously at 0.8 mg/kg on day −10, and subsequently adjusted to an area under the curve of 800 to 1200, for a total of 16 doses every 6 hours from day −9 to day −6, and cyclophosphamide was given at 60 mg/kg/day of ideal body weight on days −5 and −4.
Supportive care
Supportive care, including prophylactic antibiotics, antifungal therapy, total parenteral nutrition, hematopoietic growth factors, immune globulin replacement, and treatment of mucositis and neutropenic fever, was administered in accordance with institutional standard practice guidelines.18 Choice of antifungal agent was at the discretion of the treating physician. Sixty-six patients received Abelcet at (1-2 mg/kg), 10 received micafungin, 9 received caspofungin, and 1 patient received itraconazole. Voriconazole was prohibited as a prophylactic drug. Monitoring for CMV viremia was performed twice a week from day +21 to day +100, using polymerase chain reaction and/or the shell vial method, and patients received preemptive ganciclovir at onset of CMV reactivation.
Statistical analysis
Overall survival (OS), disease-free survival (DFS), time to relapse, acute GVHD, and NRM rates were calculated using the product-limit method, and 95% confidence intervals were calculated using the logit transformation and the Greenwood variance estimate.19 Differences between survival curves were assessed by the log-rank test. When analyzing the prognostic significance of known/suspected risk factors on time to event variables (eg, OS, DFS, relapse, NRM, acute GVHD, and TMA), Cox regression was used.20 Factors evaluated for association with outcome included: patient age at transplantation, donor sex, sex combinations (female donor to male recipient vs others; and previously pregnant female donor with male recipient vs others), disease risk status at transplantation (based on disease features and disease status at transplantation), recipient CMV status, number of CD34+ cells infused (above or below median), and conditioning regimen (Flu/Mel vs TBI/VP16 vs Bu/Cy; and reduced intensity vs ablative). Acute GVHD (grade II to IV) was treated as a time-dependent covariate in the risk factor analysis for TMA. The cumulative incidence of acute GVHD and hazard ratio were estimated after taking into account the competing risk of death and relapse. Similarly, the cumulative incidence of TMA and hazard ratio were estimated after accounting for the competing risk of death and relapse. The cumulative incidence for relapse was also computed treating a nonrelapse death event as a competing risk.
Results
Patient characteristics
Patient, disease, and treatment characteristics are shown in Table 1. The median age was 48 years (range, 10-67 years). Of the 85 patients treated, 3 (3.5%) were pediatric cases, under the age of 18 at the time of transplant. Most patients received peripheral stem cells (n = 80: 94%). The most common diagnoses were acute myeloid leukemia (39%) and acute lymphoblastic leukemia (21%). Low risk disease, defined as acute leukemia in first remission, chronic myelogenous leukemia in chronic phase, myeloproliferative disorders, and low-grade myelodysplastic syndrome (RA/RARS), was present in 54% of all cases. Disease characteristics stratified by conditioning regimen are shown in Table 2. The most commonly used regimen was Flu/Mel (54%), followed by TBI/VP16 (33%). The Bu/Cy arm was closed to accrual after a preplanned interim analysis of the first 11 patients showed a high rate of thrombotic microangiopathy (see “Toxicity/thrombotic microangiopathy” below); most of these patients had low-risk disease. Among the pediatric cases, 2 of 3 received an ablative regimen of TBI/VP16, and none of the pediatric patients was diagnosed with TMA or acute GVHD.
Patient characteristics (n = 85)
| Median age, y (range) . | 48 (10-67) . |
|---|---|
| Patient sex | |
| Female | 42 (49%) |
| Male | 43 (51%) |
| Patient/donor sex | |
| Sex match | 43 (50.6%) |
| Female/male | 21 (24.7%) |
| Male/female | 21 (24.7%) |
| Disease risk* | |
| High | 39 (46%) |
| Low | 46 (54%) |
| Diagnosis | |
| AML | 33 (39%) |
| ALL | 18 (21%) |
| non-Hodgkin lymphoma | 11 (13%) |
| MDS | 6 (7%) |
| CML | 5 (6%) |
| Hodgkin lymphoma | 4 (5%) |
| Myeloproliferative disorder | 4 (5%) |
| Multiple myeloma | 3 (3%) |
| CLL | 1 (1%) |
| Recipient CMV serostatus positive/negative | 64/21 |
| Prior autologous transplant | 6 (7%) |
| Stem cell source | |
| Bone marrow | 5 (6%) |
| Peripheral blood | 80 (94%) |
| CD34+ cell dose x106/kg: median (range) | 5.1 (1.7-10.5) |
| Conditioning regimen | |
| Flu/Mel | 46 (54%) |
| TBI/VP16 | 28 (33%) |
| Bu/Cy | 11 (13%) |
| Median age, y (range) . | 48 (10-67) . |
|---|---|
| Patient sex | |
| Female | 42 (49%) |
| Male | 43 (51%) |
| Patient/donor sex | |
| Sex match | 43 (50.6%) |
| Female/male | 21 (24.7%) |
| Male/female | 21 (24.7%) |
| Disease risk* | |
| High | 39 (46%) |
| Low | 46 (54%) |
| Diagnosis | |
| AML | 33 (39%) |
| ALL | 18 (21%) |
| non-Hodgkin lymphoma | 11 (13%) |
| MDS | 6 (7%) |
| CML | 5 (6%) |
| Hodgkin lymphoma | 4 (5%) |
| Myeloproliferative disorder | 4 (5%) |
| Multiple myeloma | 3 (3%) |
| CLL | 1 (1%) |
| Recipient CMV serostatus positive/negative | 64/21 |
| Prior autologous transplant | 6 (7%) |
| Stem cell source | |
| Bone marrow | 5 (6%) |
| Peripheral blood | 80 (94%) |
| CD34+ cell dose x106/kg: median (range) | 5.1 (1.7-10.5) |
| Conditioning regimen | |
| Flu/Mel | 46 (54%) |
| TBI/VP16 | 28 (33%) |
| Bu/Cy | 11 (13%) |
AML indicates acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; and CLL, chronic lymphocytic leukemia.
Low-risk disease is defined as acute leukemia in first remission, chronic myeloid leukemia in chronic phase, myeloproliferative disorders, and low-grade myelodysplastic syndrome (RA/RARS).
Diagnosis, remission status by conditioning regimen
| . | Flu/Mel (n = 46) . | TBI/VP16 (n = 28) . | Bu/Cy (n = 11) . |
|---|---|---|---|
| ALL | 4 (2CR, 2A) | 14 (12CR, 2A) | |
| AML | 19 (15CR, 4A) | 12 (8CR, 4A) | 2 (2A) |
| CML | 5 (4CP, 1AP) | ||
| Lymphoma/CLL | 14 (14A) | 2 (1CR, 1A) | |
| MDS | 2 (2A) | 4 (3CR, 1A) | |
| Multiple myeloma | 3 (3A) | ||
| Myeloproliferative disorder | 4 (4CP) |
| . | Flu/Mel (n = 46) . | TBI/VP16 (n = 28) . | Bu/Cy (n = 11) . |
|---|---|---|---|
| ALL | 4 (2CR, 2A) | 14 (12CR, 2A) | |
| AML | 19 (15CR, 4A) | 12 (8CR, 4A) | 2 (2A) |
| CML | 5 (4CP, 1AP) | ||
| Lymphoma/CLL | 14 (14A) | 2 (1CR, 1A) | |
| MDS | 2 (2A) | 4 (3CR, 1A) | |
| Multiple myeloma | 3 (3A) | ||
| Myeloproliferative disorder | 4 (4CP) |
AML indicates acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; and CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; A, active; AP, accelerated phase; CR, complete remission; and CP, chronic phase.
Drug levels
Tacrolimus and sirolimus serum levels were measured at least weekly (twice a week in most cases) until around day 100. During the first 30 days after transplantation, 792 samples for tacrolimus and 606 samples for sirolimus were taken from 85 patients. Median sampling points during the first 30 days were 9 for tacrolimus (range, 4-12) and 7 for sirolimus (range, 4-10). Four of 85 patients had to discontinue tacrolimus and/or sirolimus early after HCT (before day +30) due to TMA/diffuse alveolar hemorrhage, mental status change, renal insufficiency, or TMA. We calculated median levels of tacrolimus and sirolimus for the first 30 days in each patient. The median (interquartile range, IQR) tacrolimus and sirolimus levels for all patients during the first 30 days were 9.2 ng/mL (IQR, 7.8, 10.3) and 7.2 ng/mL (IQR, 5.9, 8.9), respectively. While there were no significant differences in the sirolimus levels according to the 3 conditioning regimens, the tacrolimus levels were higher in Bu/Cy and TBI/VP16 compared with Flu/Mel (Table 3).
Median tacrolimus and sirolimus levels during the first 30 days after HCT
| . | Median tacrolimus level, ng/mL (IQR) . | P . | Median sirolimus level, ng/mL (IQR) . | P . |
|---|---|---|---|---|
| Flu/Mel (n = 46) | 8.65 (6.90, 9.90)* | .003 | 7.25 (5.80, 8.70) | .916 |
| TBI/VP16 (n = 28) | 9.45 (8.20, 10.55)* | 7.00 (5.45, 9.35) | ||
| Bu/Cy (n = 11) | 10.40 (9.20, 10.99)* | 6.90 (6.20, 8.90) |
| . | Median tacrolimus level, ng/mL (IQR) . | P . | Median sirolimus level, ng/mL (IQR) . | P . |
|---|---|---|---|---|
| Flu/Mel (n = 46) | 8.65 (6.90, 9.90)* | .003 | 7.25 (5.80, 8.70) | .916 |
| TBI/VP16 (n = 28) | 9.45 (8.20, 10.55)* | 7.00 (5.45, 9.35) | ||
| Bu/Cy (n = 11) | 10.40 (9.20, 10.99)* | 6.90 (6.20, 8.90) |
HCT indicates hematopoietic cell transplantation; and IQR, interquartile range.
P < .01.
Engraftment and chimerism
All patients experienced neutrophil engraftment (absolute neutrophil count > 500) with a median time to engraftment of 15 days (range, 10-26 days). Chimerism of the day 30 bone marrow mononuclear cells by short tandem repeat analysis showed more than 90% donor in 90% of the patients.
GVHD
The probability of developing grade II to IV acute GVHD was 43% (95% CI, 37-50), with the median onset of 19 days (range, 3-89 days). Of the 34 patients who developed grade II to IV acute GVHD, 21 (25%) were grade II, 10 (12%) were grade III, and 3 (4%) were grade IV. Thirteen of 21 patients with grade II acute GVHD had mainly upper gastrointestinal (GI) involvement. Nine cases were diagnosed by histology from upper GI endoscopy (5 upper GI involvement only, 4 with both upper and lower GI tract involvement, but with limited diarrhea < 500 mL). Four cases without a biopsy had significant upper GI tract symptoms (nausea, vomiting, and anorexia), and either stage I to II skin, stage 0 liver (alanine aminotransferase/aspartate aminotransferase [ALT/AST] elevation), or stage 0 lower GI GVHD (mild diarrhea < 500 mL) for which steroids were started. In these 13 cases of mainly upper gastrointestinal GVHD, the median onset was 23 days (range, 11-85 days).
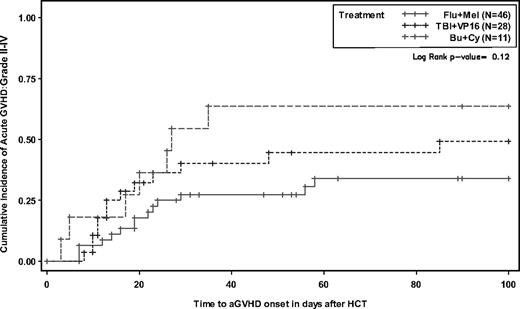
There was a higher probability of acute GVHD for patients conditioned with Bu/Cy compared with TBI/VP16 and Flu/Mel (64%, 49%, and 34%, respectively) without reaching statistical significance (P = .12; Figure 1). The probability of limited and extensive chronic GVHD was 51% (29% limited, 71% extensive).
Cumulative incidence of grade II to IV acute graft-versus-host disease according to conditioning.
Cumulative incidence of grade II to IV acute graft-versus-host disease according to conditioning.
Survival
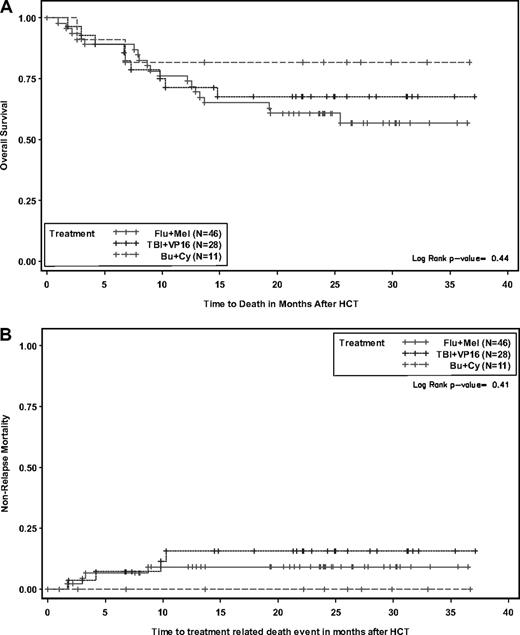
With a median follow-up of 26 months (range, 14-37 months) for surviving patients, 55 of the 85 patients are alive. Twenty-two patients died of relapse, while 8 deaths were due to nonrelapse causes, including acute/chronic GVHD (3), multiorgan failure (1), mucormycosis (1), leukoencephalopathy (1), multifactorial respiratory failure developing after initial diffuse alveolar hemorrhage (1), and unknown cause without relapse (1). The probabilities of OS, DFS, and relapse at 2 years were 66% (CI, 59%-72%), 58% (CI, 52%-64%), and 34% (CI, 28%-42%), respectively. The day-100, 1-year, and 2-year NRM rates were 4.8% (CI, 2%-12%), 10.2% (CI, 6%-18%), and 10.2% (CI, 6%-18%), respectively. Conditioning regimen was not significantly associated with OS (Figure 2A), DFS, relapse, or NRM (Figure 2B).
Probabilities according to conditioning. Overall survival (A) and nonrelapse mortality (B).
Probabilities according to conditioning. Overall survival (A) and nonrelapse mortality (B).
Toxicity/thrombotic microangiopathy
Patients who experienced grade III or higher lung, liver, and TMA toxicity are summarized in Table 4 by conditioning regimen. Two cases of fatal alveolar hemorrhage were seen, 1 with Flu/Mel and 1 with TBI/VP16. Engraftment syndrome, characterized by fever, rash, and pulmonary infiltrates around the time of engraftment, was frequently observed with Flu/Mel (22%), but was reversible with corticosteroids in all cases. Liver toxicity (CTCAE v3.0, more than grade III) including VOD (n = 2) was seen more frequently with Bu/Cy (27%) than with TBI/VP16 (18%) or Flu/Mel (11%), without statistical significance (Table 4); all cases were reversible. The non-VOD liver toxicities were mostly elevated liver enzymes without clear GVHD and noted at the median onset of 23 days after HCT (range, day −4 to +76).
Lung, liver, TMA toxicities, grade more than III by regimen
| . | Lung . | P* . | Liver . | P* . | TMA . | P* . |
|---|---|---|---|---|---|---|
| Flu/Mel (n = 46) | 10 (22%) | .087 | 5 (11%) | .305 | 3 (7%) | < .005 |
| TBI/VP16 (n = 28) | 1 (4%) | 5 (18%) | 7 (25%) | |||
| Bu/Cy (n = 11) | 1 (9%) | 3 (27%) | 6 (55%) |
| . | Lung . | P* . | Liver . | P* . | TMA . | P* . |
|---|---|---|---|---|---|---|
| Flu/Mel (n = 46) | 10 (22%) | .087 | 5 (11%) | .305 | 3 (7%) | < .005 |
| TBI/VP16 (n = 28) | 1 (4%) | 5 (18%) | 7 (25%) | |||
| Bu/Cy (n = 11) | 1 (9%) | 3 (27%) | 6 (55%) |
Fisher exact test.
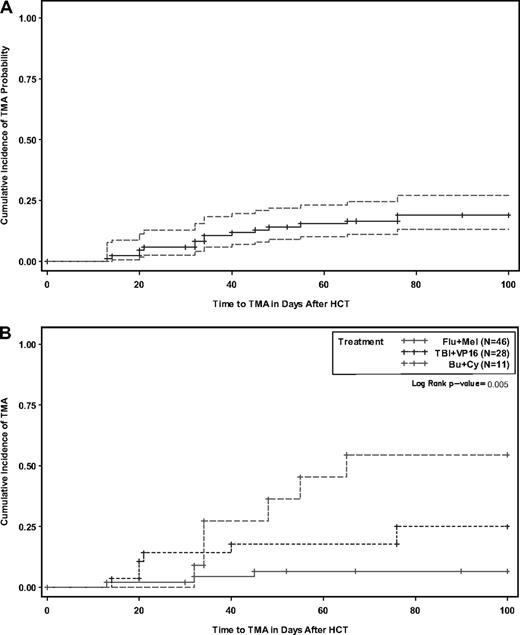
TMA was a major complication that developed in 16 patients (19%). The median time to onset was 5 weeks after HCT (Figure 3A-B) and was found to be significantly associated with conditioning regimen (Table 4): Bu/Cy (55%) compared with TBI/VP16 (25%) and Flu/Mel (6.5%; P < .005). The Bu/Cy arm was closed to accrual early as a result of this finding. Twelve of 16 patients with TMA were also diagnosed with acute GVHD. GVHD preceded TMA in all of these cases. TMA was managed by holding tacrolimus in 50% of cases and/or holding sirolimus in 85% of cases; plasma exchange was performed in 1 case. Hemodialysis was required in 1 patient. TMA was reversible in all cases. The median levels (ng/mL) of tacrolimus and sirolimus at onset of TMA were 9.4 (range, 6.7-13.0) and 8.1 (range, 5.3-13.3), respectively, not significantly different from the median values for non-TMA patients over the first 30 days (tacrolimus: 9.1 [range, 3.5-13.4], P = .14; sirolimus: 7.2 [range, 2.5-18.8], P = .33). We further examined the effect of drug level on TMA risk using cut points at each quartile. Although TMA risk appeared to increase incrementally with increasing drug exposure by quartile analysis (Table 5), none of the cut point classifications (ie, quartiles; <, ≥ 25th percentile; median; <, ≥ 75th percentile) for tacrolimus or sirolimus were significantly associated with TMA risk. In addition, an examination of a possible threshold effect between serum level and onset of TMA was explored using the generalized estimating equation method. While the results of this analysis also showed no threshold effect, the results were difficult to estimate given the limited number of patients and TMA events.
Cumulative incidence with 95% confidence interval. Thrombotic microangiopathy (A) and stratified by conditioning (B).
Cumulative incidence with 95% confidence interval. Thrombotic microangiopathy (A) and stratified by conditioning (B).
Results of Cox univariate and multivariate analysis for TMA
| Value . | N . | No. of TMA . | Univariate hazard rate ratio (95% CI) . | Univariate P . | Multivariate hazard rate ratio (95% CI) . | Multivariate P . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| Less than 48 | 44 | 9 | Baseline | * | ||
| 48 or older | 41 | 7 | 0.8 (0.3-2.16) | 0.66 | ||
| Patient/donor sex match | ||||||
| Others | 64 | 12 | Baseline | * | ||
| Male patient/female donor | 21 | 4 | 0.99 (0.32-3.07) | 0.99 | ||
| Disease risk | ||||||
| Low | 47 | 12 | Baseline | Baseline | 0.18 | |
| High | 38 | 4 | 0.39 (0.13-1.21) | 0.10 | 0.46 (0.14-1.44) | |
| Conditioning regimen | ||||||
| Flu/Mel | 46 | 3 | Baseline | Baseline | 0.03 | |
| TBI/VP16 and Bu/Cy | 39 | 13 | 5.61 (1.6-19.68) | 0.007 | 4.03 (1.12-14.42) | |
| Patient CMV serology | ||||||
| Negative | 21 | 3 | Baseline | * | ||
| Positive | 64 | 13 | 1.47 (0.42-5.17) | 0.55 | ||
| CD34 cell dose | ||||||
| Less than 5.1 | 44 | 8 | Baseline | * | ||
| 5.1 or more | 41 | 8 | 1.03 (0.39-2.75) | 0.95 | ||
| Acute GVHD | ||||||
| Grade 0 to I | 51 | 4 | Baseline | Baseline | 0.02 | |
| Grade II to IV | 34 | 12 | 5.00 (1.61-12.53) | 0.005 | 3.89 (1.24-12.28) | |
| Tacrolimus† | ||||||
| Less than 25th (< 7.8) | 22 | 2 | Baseline | * | ||
| 25th to 50th (7.8-9.2) | 20 | 4 | 2.20 (0.40, 12.0) | 0.36 | ||
| 50th to 75th (9.3-10.3) | 21 | 4 | 1.86 (0.34, 10.15) | 0.47 | ||
| More than 75th (> 10.3) | 21 | 6 | 3.10 (0.63, 15.38) | 0.17 | ||
| Sirolimus† | ||||||
| Less than 25th (< 5.9) | 22 | 3 | Baseline | * | ||
| 25th to 50th (5.9-7.2) | 23 | 4 | 1.29 (0.29, 5.76) | 0.74 | ||
| 50th to 75th (7.3-8.9) | 19 | 4 | 1.56 (0.35, 6.95) | 0.56 | ||
| More than 75th (> 8.9) | 21 | 5 | 1.82 (0.43, 7.61) | 0.41 |
| Value . | N . | No. of TMA . | Univariate hazard rate ratio (95% CI) . | Univariate P . | Multivariate hazard rate ratio (95% CI) . | Multivariate P . |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| Less than 48 | 44 | 9 | Baseline | * | ||
| 48 or older | 41 | 7 | 0.8 (0.3-2.16) | 0.66 | ||
| Patient/donor sex match | ||||||
| Others | 64 | 12 | Baseline | * | ||
| Male patient/female donor | 21 | 4 | 0.99 (0.32-3.07) | 0.99 | ||
| Disease risk | ||||||
| Low | 47 | 12 | Baseline | Baseline | 0.18 | |
| High | 38 | 4 | 0.39 (0.13-1.21) | 0.10 | 0.46 (0.14-1.44) | |
| Conditioning regimen | ||||||
| Flu/Mel | 46 | 3 | Baseline | Baseline | 0.03 | |
| TBI/VP16 and Bu/Cy | 39 | 13 | 5.61 (1.6-19.68) | 0.007 | 4.03 (1.12-14.42) | |
| Patient CMV serology | ||||||
| Negative | 21 | 3 | Baseline | * | ||
| Positive | 64 | 13 | 1.47 (0.42-5.17) | 0.55 | ||
| CD34 cell dose | ||||||
| Less than 5.1 | 44 | 8 | Baseline | * | ||
| 5.1 or more | 41 | 8 | 1.03 (0.39-2.75) | 0.95 | ||
| Acute GVHD | ||||||
| Grade 0 to I | 51 | 4 | Baseline | Baseline | 0.02 | |
| Grade II to IV | 34 | 12 | 5.00 (1.61-12.53) | 0.005 | 3.89 (1.24-12.28) | |
| Tacrolimus† | ||||||
| Less than 25th (< 7.8) | 22 | 2 | Baseline | * | ||
| 25th to 50th (7.8-9.2) | 20 | 4 | 2.20 (0.40, 12.0) | 0.36 | ||
| 50th to 75th (9.3-10.3) | 21 | 4 | 1.86 (0.34, 10.15) | 0.47 | ||
| More than 75th (> 10.3) | 21 | 6 | 3.10 (0.63, 15.38) | 0.17 | ||
| Sirolimus† | ||||||
| Less than 25th (< 5.9) | 22 | 3 | Baseline | * | ||
| 25th to 50th (5.9-7.2) | 23 | 4 | 1.29 (0.29, 5.76) | 0.74 | ||
| 50th to 75th (7.3-8.9) | 19 | 4 | 1.56 (0.35, 6.95) | 0.56 | ||
| More than 75th (> 8.9) | 21 | 5 | 1.82 (0.43, 7.61) | 0.41 |
TMA indicates thrombotic microangiopathy; CI, confidence interval; CMV, cytomegalovirus; and GVHD, graft-versus-host disease.
Not tested.
Patient's 30-day median value, by quartile.
CMV infection
CMV reactivation was observed in 16 of 64 patients (25%) who were seropositive. There were no cases of CMV disease (CMV pneumonia or gastrointestinal involvement).
Factors associated with outcome
Cox regression analysis was performed to identify potential factors (see “Statistical analysis” above) associated with survival, relapse, NRM, acute GVHD, and TMA. For the survival/relapse endpoints, the patient disease risk (high or low) was the primary factor significantly associated with OS, DFS, relapse risk, and NRM. In addition, tacrolimus/sirolimus levels over the first 30 days were included in the model in evaluating the risk factors for acute GVHD and TMA (Table 5). There were no significant factors found to predict acute GVHD in our analysis. Significant factors associated with increased TMA risk were conditioning regimen (patients conditioned with an ablative regimen, either TBI or Bu/Cy, had a 5-fold increase in risk) and prior acute GVHD (grade II-IV), which both remained significant in multivariate analysis (Table 5).
Discussion
Based on encouraging phase II study data from DFCI,8,9 we prospectively tested the combination of tacrolimus and sirolimus as GVHD prophylaxis in patients undergoing HLA-matched sibling donor HCT conditioned with 3 different regimens. Administration of conditioning therapy and GVHD prophylaxis were nonoverlapping to avoid potential adverse interactions.
In our study, we observed a 43% rate of grade II to IV acute GVHD (grade III-IV, 19%). The data are at least comparable with our historic experience; the incidence of acute GVHD grade II to IV was approximately 50% in Flu/Mel conditioning using cyclosporine and micophenolate,21 and approximately 60% in full-intensity conditioning (TBI/VP, TBI/Cy, Bu/Cy) using cyclosporine and methorexate.22 The incidence of acute GVHD was lowest with Flu/Mel followed by TBI/VP16, and the highest in Bu/Cy, although the difference did not reach statistical significance (P = .12), as sample size for the Bu/Cy group was small due to early closure.
The DFCI experience with tacrolimus and sirolimus showed a lower cumulative incidence of grade II to IV acute GVHD of 19% using a conditioning regimen of cyclophosphamide followed by TBI,8,9 and 11% using the reduced intensity regimen of intravenous busulfan and fludarabine.23 The reason for the relatively higher rate of acute GVHD in this study is unknown. The patient characteristics in our study appear similar to those reported by DFCI. The difference may be partly attributable to different conditioning regimens used in our study (FTBI/Cy vs FTBI/VP16, Bu/Cy, and Flu/Mel). The FHCC recently published their experience using a calcineurin inhibitor with sirolimus and methotrexate for 26 high-risk patients receiving unrelated donor transplants, conditioned with TBI/Cy or Bu/Cy. A high incidence of grade II to IV acute GVHD of 77% led to the early discontinuation of the study.13 Suggested possible explanations for the discrepant results between DFCI and FHCC include different timing of immunosuppression, use of cyclosporine instead of tacrolimus in some cases, and use of Bu/Cy in some cases.24
Of clinical importance is the fact that severity of acute GVHD in our experience was generally mild; for example, approximately 60% of cases with grade II GVHD were mainly upper gastrointestinal, a form of GVHD that is known to have a relatively good prognosis.25 It has been recognized that conditioning-related injury of the upper GI tract can be clinically and histologically similar to upper gastrointestinal GVHD if diagnosed close to conditioning therapy. This may possibly explain the higher rate of acute GVHD in this study compared with DFCI results. Overall, 3 deaths (of 85 patients) were directly attributable to GVHD. When analyzed as a time-dependent variable, acute GVHD (grade II to IV) was not significantly associated with inferior OS or NRM in our cohort (data not shown). Chronic GVHD observed in our study was similar to the DFCI results.
Our survival data were very encouraging with a remarkably low NRM rate of 10% over 2-year follow-up across the 3 conditioning regimens, suggesting that most of the toxicities were reversible. In this study the major cause of treatment failure was relapse. Although the use of sirolimus was recently suggested to be protective from relapse in lymphoid malignancy,26 further modifications in HCT protocols need to be explored to reduce the relapse risk.
TMA was a major complication in our study that developed in 19% of patients, consistent with an earlier report.12 TMA was significantly associated with Bu/Cy conditioning, which led to the early discontinuation of that regimen from the trial. Increased hepatotoxicity/VOD associated with Bu/Cy in our study is also consistent with a recent report from DFCI showing an increased incidence of VOD in patients conditioned with Bu/Cy in their tacrolimus plus sirolimus GVHD prophylaxis.12 In the ongoing BMT CTN Protocol 0402 (available at https://web.emmes.com/study/bmt2) comparing tacrolimus plus sirolimus versus tacrolimus plus methotrexate for matched related transplants, an increased incidence of VOD in patients conditioned with Bu/Cy in the tacrolimus plus sirolimus arm has led to the early discontinuation of that conditioning regimen.
The higher incidence of TMA/VOD with Bu/Cy is possibly related to the known endothelial toxicity of the conditioning regimen,27,28 coupled with the synergistic effects on canalicular and sinusoidal transport proteins and reduced glutathione–related enzymes from calcineurin inhibitors and sirolimus, leading to reduced protection against oxidative stress and possibly leading to accumulation of toxic metabolites in the hepatocytes.29 We observed higher tacrolimus levels in Bu/Cy compared with Flu/Mel, but not with TBI/VP16 (as shown in Table 3). The tacrolimus levels were not significantly associated with TMA in our univariate analysis; thus, it is unlikely that the increased risk of TMA in Bu/Cy was attributable to the tacrolimus levels. While this analysis showed no threshold effect of the drug levels, the results were difficult to estimate given the limited number of patients and TMA events.
The relationship of GVHD and TMA is difficult to define from this limited experience, although there was a significant association between acute GVHD (grade II to IV) and TMA. Twelve of 16 cases with TMA had acute GVHD, and GVHD anteceded TMA in all cases. This suggests that optimization of tacrolimus and sirolimus dosing may have led to higher drug levels, potentially leading to TMA in addition to ongoing tissue damage from the GVHD itself. Thus, more careful dose adjustment, particularly in the setting of acute GVHD, may reduce the risk of TMA and toxicity profile, although these data do not demonstrate a clear association between the drug levels and TMA, possibly due to the small sample size.
In univariate and multivariate analysis, the most significant factor predicting OS, DFS, relapse, and NRM was disease status. We found no clinical variables significantly predictive for development of grade II to IV acute GVHD within our cohort. Conditioning regimen was not significantly associated with OS or DFS, despite higher incidence of TMA and a trend toward higher incidence of GVHD in Bu/Cy. This may be partly explained by the fact that a majority of patients in this group had good-risk disease. The univariate/multivariate analysis data need to be interpreted with caution, however, because of the heterogeneity of diseases, disease status, and conditioning regimens.
In conclusion, the combination of tacrolimus and sirolimus as GVHD prophylaxis was associated with an excellent NRM rate across multiple conditioning regimens, lending strong support to the ongoing phase III BMT CTN trial 0402. Acute GVHD rates, while higher than those seen by DFCI, were reasonable for the Flu/Mel and TBI/VP16 regimens, with most cases relatively mild and well tolerated. Given the high incidence of associated TMA, tacrolimus/sirolimus prophylaxis must be used with caution in patients conditioned with Bu/Cy and potentially with other regimens likely to cause increased endothelial damage.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the transplant coordinators and transplant nurses for their dedicated care of our patients. We also thank all members of the Hematopoietic Cell Transplant team for their constant support of the program, including the clinical research associates for their protocol management and data collection support. We also thank our scientific writer/editor Dr Sandra Thomas for her critical reading of the manuscript.
This work was supported by the Comprehensive Cancer Center grant (P30 CA 33572), Hem/HCT Program Project grant (PO1 CA 30206-21) and the Tim Nesvig fellowship, which R.N. was recently awarded.
National Institutes of Health
Authorship
Contribution: R.R., R.N., J.M.P., P.P., A.N., and S.J.F. conceived and designed the study; R.R., R.N., P.P., S.S., A.N., D. Snyder, V.P., N.K., E.S., C.K., M.O., A.Y.K., D. Senitzer, and S.J.F. provided study materials or patients; R.R., R.N., J.M.P., P.P., S.S., A.N., D. Snyder, V.P., N.K., J.R., E.S., C.K., M.O., A.Y.K., D. Senitzer, and S.J.F. provided administrative, technical, or logistical support; R.R., R.N., J.M.P., P.P., S.S., A.N., D. Snyder, V.P., N.K., J.R., E.S., C.K., M.O., A.Y.K., D. Senitzer, and S.J.F. collected and assembled the data; R.R., R.N., J.M.P., P.P., S.S., and S.J.F. analyzed and interpreted the data; and R.R., R.N., J.M.P., P.P., S.S., and S.J.F. drafted the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ryotaro Nakamura, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: rnakamura@coh.org.
References
Author notes
R.R. and R.N. contributed equally to this work.